Uremic toxins (UTs) are mainly produced by protein metabolized by the intestinal microbiota and converted in the liver or by mitochondria or other enzymes. The accumulation of UTs can damage the intestinal barrier integrity and cause vascular damage and progressive kidney damage. Together, these factors lead to metabolic imbalances, which in turn increase oxidative stress and inflammation and then produce uremia that affects many organs and causes diseases including renal fibrosis, vascular disease, and renal osteodystrophy. This article is based on the theory of the intestinal–renal axis, from bench to bedside, and it discusses nonextracorporeal therapies for UTs, which are classified into three categories: medication, diet and supplement therapy, and complementary and alternative medicine (CAM) and other therapies. The effects of medications such as AST-120 and meclofenamate are described. Diet and supplement therapies include plant-based diet, very low-protein diet, probiotics, prebiotics, synbiotics, and nutraceuticals. The research status of Chinese herbal medicine is discussed for CAM and other therapies. This review can provide some treatment recommendations for the reduction of UTs in patients with chronic kidney disease.
- chronic kidney disease
- diet control
- dietary supplement
- complementary and alternative medicine
- uremic toxin
- conventional medical therapy
1. Introduction
Chronic kidney disease (CKD) is characterized by a gradual decrease in the glomerular filtration rate and proteinuria. CKD is a global health problem, and its incidence has been increasing. The estimated global prevalence of CKD is 8–14% [1,2][1][2]. When kidney function deteriorates gradually, many metabolites accumulate in the body. These accumulated substances, termed as uremic toxins (UTs), can result in adverse pathophysiological outcomes [3]. UTs can affect multiple organs and cause renal fibrosis, vascular calcification, anemia, peripheral arterial disease [4], adynamic bone disease [5], adipocyte dysfunction with insulin resistance [6], impaired immune system [7], uremic pruritus [8], and impaired valsartan-induced neovascularization [9].
In 1999, Vanholder and other researchers established the European Uremic Toxin (EUTox) Work Group and divided UTs into three categories based on their solubility, molecular weight, and ability to bind to serum proteins. These categories are small water-soluble compounds such as creatinine and uric acid (UA), middle molecules such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β, and compounds that bind to proteins such as indoxyl sulfate (IS) [10]. The EUTox Work Group continues to identify new UTs and specify their standard and uremic concentrations [3]. Esmeralda et al. revised some UTs items based on several inflammatory markers that would increase the deterioration of CKD [11]. Currently, many hundreds of UTs including small water-soluble solutes, which can be removed through dialysis, and larger and protein-bound molecules, which are less likely to be removed during dialysis [12]. The pathophysiological mechanisms through which UTs cause multiple organ damage are complex and not completely understood. These mechanisms may include inflammation, reactive oxidative stress, cellular transdifferentiation, impaired mitochondria function, intestinal barrier destruction, and changes in intestinal microbiota [12,13,14][12][13][14]. Figure 1 shows the proposed mechanism of UTs generation. After food enters the intestine, it is not only digested by exocrine glands such as the liver, gallbladder, intestines, and pancreas but also decomposed by intestinal microbiota. Protein catabolism produces amino acids. If many ingredients that are favored by harmful intestinal microbiota are consumed, including tryptophan and tyrosine, then they will be converted into UTs through the proteolysis of intestinal microbiota [13,15][13][15]. Precursors such as indole and p-cresol are absorbed by human intestinal villi cells into the portal blood circulation of the liver. Subsequently, the liver metabolizes them into IS and p-tolyl sulfate [16]. CKD patients are more prone to constipation than ordinary people. The retention of feces will change the intestinal microbiota and promote the production and accumulation of more UTs. When the anaerobic bacteria that can produce short-chain fatty acids (SCFAs) are reduced, SCFAs such as butyrate and acetate, that promote intestinal contraction is also reduced, which in turn makes constipation worse [17,18][17][18]. The accumulation of UTs destroys the protective barrier of the intestinal epithelium, leading to the transfer of microbiota from the intestine to the body [13,19][13][19]. After IS produced in the liver enters the blood circulatory system, which is absorbed by proximal renal tubular cells through organic anion transporters (OAT) 1 and OAT3 at the basolateral membrane. The accumulated IS induces oxidative stress and then activates nuclear factor E2-related factor 2 (Nrf2) [20]. Some of these UTs can destroy mitochondrial function. Mitochondria may also be the source of UTs because mitochondria contain certain enzymes involved in the UTs synthesis pathway; thus, they may be the site of UTs synthesis. Moreover, because mitochondria are the key regulators of cellular redox homeostasis, they may directly affect the production of UTs. In addition, because many metabolites can be degraded in mitochondria, mitochondrial dysfunction might cause the accumulation of this toxin in organisms. Therefore, CKD can lead to the appearance of compounds that destroy mitochondria, and subsequent mitochondrial damage can cause further accumulation of UTs; the synthesis of UTs is related to mitochondria [14]. CKD reduces the ability to excrete UTs, leading to the accumulation of UTs in blood. At the same time, UTs can accelerate the deterioration of kidney function, leading to a vicious circle. All these factors together lead to the typical destruction of normal metabolic balance and uremic homeostasis that results in inflammation and uremia, causing multiple organ damage [12]. According to the review articles, due to the use of a highly permeable membrane with a greater pores radius and better preservation of the residual renal function, peritoneal dialysis (PD) could be anticipated that some uremic toxins are more efficiently cleared across the peritoneal membrane [21], and that the plasma levels of p-Cresol (protein-bound uremic toxin) are lower than in hemodialysis patients [22].
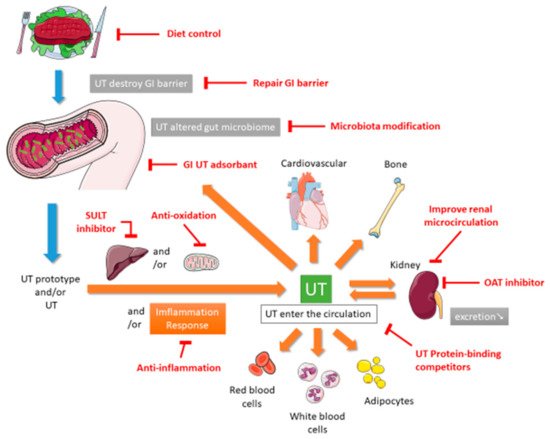
This review describes the potential nonextracorporeal methods from bench to bedside that can be used to reduce UTs levels and improve renal function based on the aforementioned mechanisms. The PubMed, Embase, Cochrane Library, Chinese National Knowledge Infrastructure, Airiti library, and Wanfang databases were searched by using the term “uremic toxin”. To broaden the search, we further reviewed the included articles and citations utilizing the “related articles” facility on PubMed. This paper is organized as follows. The first section focuses on medications including intestinal sorbents, UA modulators, and enzyme inhibitors. The second section focuses on diet control and supplement therapies such as probiotics, prebiotics, synbiotics, and nutraceuticals. The final section describes complementary and alternative medicine (CAM) and other therapies.
2. Conventional Medication Therapy
In conventional medical therapies, the serum concentration of UTs is mainly reduced using drugs that control underlying diseases to slow down the deterioration of the kidneys, like decrease hypertension, neutralize catecholamines, combat fluid overload, combat dyslipidemia and anemia [23], exert antioxidative effects [24], cause the metabolic degradation of UTs [25], change the bacterial amino acid metabolism [26], inhibit UA synthesis [27], inhibit sulfotransferase [28], reduce amino acid degradation [29], inhibit renal OAT [30], and combine or reduce the intake of toxins or their precursors to adjust the intestinal absorption capacity [31]. In addition, dialysis is performed for extracorporeal removal of UTs; however, the removal of middle and protein-bound UTs through conventional dialysis is inadequate [12]. The following is a brief discussion of treatment strategies that can be used to reduce UTs levels ( Table 1 ).
| Intervention | Route, Dosage and Frequency | Author/Year | Mechanism/Usage | Study Design | Subjects | Subject Number | Result |
|---|---|---|---|---|---|---|---|
| Clinical Studies | |||||||
| Acarbose | Oral, 100 mg, TID | Evenepoel et al., 2006 [26] | Changes in bacterial amino acid metabolism | Clinical trial | Healthy people | 9 | PCS ↘ |
| AST-120 | Oral, 2.7 to 9 g/day | Chen et al., 2019 [32] | UT adsorbent | Meta-analysis | Patients with CKD | 3349 | IS ↘ |
| L-carnitine | i.v., 20 mg/kg, 3 times/week | Fatouros et al., 2010 [33] | Antioxidation | Clinical trial | Patients undergoing HD | 12 | MDA ↘ |
| Folate | Oral, 10 mg, QD | Trimarchi et al., 2002 [34] | Metabolic degradation of UT | RCT | Patients undergoing HD | 62 | Hcy ↘ |
| Folate and Methylcobami | i.v. methylcobalami 500 µg, 3 times/week and oral folate 15 mg, QD | Koyama et al., 2010 [25] | Metabolic degradation of UT | RCT | Patients undergoing HDs | 40 | ADMA ↘, Hcy ↘ |
| Ketoacid and LPD | Oral, 1 pill/5 kg, QD | Marzocco et al., 2013 [35] | Decreased amino acid degradation/protein carbamylation | RCT | CKD stage 3 adults | 32 | IS ↘ |
| Ketoacid and LPD | Oral, 0.1 g/kg, TID | Garibotto et al., 2018 [29] | Decreased amino acid degradation/protein carbamylation | RCT | Patients with CKD | 17 | Urea ↘ |
| Reduced glutathione | Oral, 400 mg, TID | Wang et al., 2016 [36] | Antioxidation | RCT | Patients undergoing HD | 150 | IL-6 ↘, TNF-α ↘ |
| Animal Studies | |||||||
| AST-120 | Oral, 8% w/w, QD | Sato et al., 2017 [37] | UT adsorbent | Animal | Adenine-induced CKD mice | 24 | IS ↘, PCS ↘ |
| L-carnitine | i.p., 500 mg/kg, QD | Sener et al., 2004 [38] | Antioxidation | Animal | Right nephrectomy rats | 16 | BUN ↘, Cr ↘, MDA ↘ |
| Cilastatin | i.v., 200 mg/kg, once | Huo et al., 2019 [30] | OAT inhibitor | Animal | Imipenem-induced nephrotoxicity rabbits | 4 | BUN ↘, Cr ↘ |
| cyclosporine | i.v., 3 mg/kg, once | Lemoine et al., 2015 [24] | Antioxidation | Animal | I/R mice | 22 | BUN ↘, Cr ↘ |
| Enalapril | Oral, 12.6 mg/kg, QD | Marek et al., 2018 [39] | ACEI, increased glomerular filtration, and urine output | Animal | Wistar rats | 27 | TMAO ↘ |
| meclofenamate | i.v., 10 mg/kg, TID | Saigo et al., 2014 [28] | SULT inhibitors | Animal | Renal I/R rats | 9 | BUN ↘, Cr ↘, IS ↘ |
| Probenecid | i.v., 50 mg/kg, once | Huo et al., 2019, [30] | OAT inhibitor | Animal | Imipenem-induced nephrotoxicity rabbits | 12 | BUN ↘, Cr ↘ |
Acarbose is a small intestinal alpha-glucosidase inhibitor that can increase the number of undigested carbohydrates reaching the colon, increase the production of short-chain fatty acid butyrate in the colon, and reduce intraluminal power of hydrogen (pH) value, thereby inhibiting bacterial deamination and increasing the use of ammonia. In a clinical trial, nine healthy people were treated with acarbose for 3 weeks, and their serum p-cresol declined significantly through changes in bacterial amino acid metabolism [26].
Patients undergoing hemodialysis (HD) may develop L-carnitine deficiency due to intestinal malabsorption, reduced carnitine synthesis, and reduced carnitine clearance during dialysis. In a double-blind, placebo-controlled, crossover trial of patients undergoing HD, supplementation of L-carnitine, an antioxidant, for 8 weeks reduced the malondialdehyde (MDA) level, increased reduced/oxidized glutathione, and enhanced glutathione peroxidase activity and protein carbonyl concentration without adverse clinical effects [33]. The intravenous injection of carnitine supplementation has been shown to have a nephroprotective effect on contrast-medium nephropathy in an open-label, crossover study, it can reduce the neutrophil gelatinase associated lipocalin and SCr [42][40]. An animal study suggested that the nephroprotective effect of L-carnitine could reduce blood urea nitrogen (BUN), Scr, and MDA levels in CKD rats following right nephrectomy, possibly through antioxidative properties and free radical scavenging [38].
Oxidative stress arises when there is an imbalance between free radical production and antioxidant defense. Uremic toxins can be a source of oxidative stress in CKD patients. Retention of these toxins promotes systemic inflammation via priming polymorphonuclear leukocytes and stimulating CD-8+ cells [47][41]. Additional associative factors of oxidative stress in CKD include low serum selenium concentration, low platelet glutathione peroxidase activity [48][42], and lower serum levels of glutathione [48][42]. Although glutathione is critical to fight against oxidative stress, some evidences disclosed that glutathione is poorly absorbed by oral route mainly due to the action of an intestinal enzyme, the γ-glutamyl transpeptidase which degrades glutathione [49][43]. Wang et al. ever found that the oral supplementation of reduced glutathione in patients undergoing HD significantly reduced their IL-6 and TNF-α levels but not their Scr or BUN levels in their RCT trial [36]. Although the very low oral bioavailability limits the interest of oral glutathione supplementation, Schmitt et al. demonstrated the superiority of a new sublingual form of glutathione over the oral form in terms of glutathione supplementation [50][44]. A new sublingual form may be a promising antioxidant therapy in CKD patients in the future.
3. Diet Control and Diet Supplements
UTs are usually produced through the metabolism of amino acids by intestinal microbiota, and accumulated UTs can change the composition of intestinal microbiota. Increasing evidence shows that intestinal microbiota plays a crucial role in the development of CKD [51][45]. Therefore, the current methods of reducing UTs through diet control and supplement therapy include reducing protein intake through a low-protein or plant-based diet [52][46] and supplementing probiotics, prebiotics, and synbiotics to change the composition of intestinal microbiota; some supplements could exert anti-inflammatory and antioxidative effects [53][47]. These treatment strategies are briefly discussed below ( Table 2 ).
Synbiotics, a combination of probiotics and prebiotics, have immune system regulation and anti-inflammatory effects. It can improve the intestinal environment and reduce concentration of nitrogen-containing metabolites [81[48][49],82], was applied to treat intestinal chronic diseases [82][49], allergic rhinitis and asthma [83][50]. An uncontrolled trial showed that a synbiotic consisting of Lactobacillus casei , Bifidobacterium breve, and galactooligosaccharides reduced the PCS level but did not change IS or phenol levels in patients undergoing HD [58][51].
Another review supports this observation and revealed that plant-based foods and Mediterranean (MD) diet or Dietary Approaches to Stop Hypertension (DASH) diets could reduce many types of UTs; however, the potential risk of hyperkaliemia should be examined [87][52]. A cohort study revealed that vegetarian diet patients in hemodiafiltration have not only lower plasma levels of IS and PCS, but also lower serum level of urea [59][53].
Case–control studies have shown that vitamin D supplementation could improve endothelial dysfunction in patients with CKD [89][54]. An RCT reported that vitamin D supplementation for 8 weeks could reduce the IL-6 and UA levels in patients with nondiabetic CKD stage 3–4 and vitamin D deficiency. In addition, vitamin D supplementation could improve vascular function, as determined by significant positive changes in pulse wave velocity [53][47].
4. Complementary and Alternative Medicine Therapy
Dahuang Fuzi Decoction (DFD), a well-known traditional Chinese prescription, consists Dahuang ( Radix et Rhizoma Rhei ), Paofuzi ( Radix Aconiti Lateralis Preparata ), and Xixin ( Radix et Rhizoma Asari ), originated from the Synopsis of Golden Chamber [96][55]. DFD is often used to treat gynecological diseases and CKD [122][56], it could also reduce BUN, Scr, and UA levels in an adenine-induced renal injury rat model. Moreover, DFD could block the activation of transforming growth factor beta 1 c-Jun N-terminal kinase (TGF-b1-JNK) pathways to mitigate renal damage and tubular epithelial apoptosis [96][55].
Osthole isolated from Cnidium moonnieri (L.) Cussion, which is a coumarin derivative, protects cerebral artery occlusion injury by exerting anti-inflammatory effects [149][57]. In an I/R-induced renal injury model, Luo et al. demonstrated that the administration of osthole reduced TNF-α, IL-8, and IL-6 levels. Osthole prevents renal injury by suppressing the Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway and activating PI3K/Akt signaling pathway [110][58].
Resveratrol (RSV), a natural polyphenol that originates in grapes, berries, and other dietary constituents, exerts antioxidant activity and anti-atherosclerotic effects by modulating the growth of certain gut microbiota, such as Lactobacillus and Bifidobacterium [152,153][59][60]. RSV can promote the release of nitric oxide and prostacyclin to maintain endothelial function and adjust vascular tone [154][61]. RCT confirmed that RSV has cardioprotective effects in patients with stable coronary artery disease [155][62]. Chen et al. reported that RSV attenuated TMAO in TMAO-induced atherosclerosis mice through the remodeling of gut microbiota [112][63].
Acupuncture is a popular CAM therapeutic method that can effectively treat certain diseases such as pain and insomnia; this method is recognized by WHO [162][64]. Acupuncture can stimulate the production of endomorphin-1, encephalin, β-endorphin, and serotonin in plasma and brain tissue, thereby achieving the effects of analgesia, sedation, and immune regulation [163][65]. In an RCT including 53 patients with CKD, once-weekly electroacupuncture at bilateral Hegu (LI4), Zusanli (ST36), and Taixi (KI3) for 12 weeks improved renal function; the reduction in Scr levels induced by reduced eGFR was greater in the acupuncture group than in the sham acupuncture group [92][66]. Acupuncture might improve renal function by regulating sympathetic nerves and activating biologically active chemicals [164][67].
References
- Coresh, J.; Selvin, E.; Stevens, L.A.; Manzi, J.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Levey, A.S. Prevalence of chronic kidney disease in the United States. JAMA 2007, 298, 2038–2047.
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.; Bhave, N.; Bragg-Gresham, J.; Balkrishnan, R.; Dietrich, X.; Eckard, A.; Eggers, P.W. US renal data system 2017 annual data report: Epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2018, 71, A7.
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270.
- Hung, S.C.; Kuo, K.L.; Huang, H.L.; Lin, C.C.; Tsai, T.H.; Wang, C.H.; Chen, J.W.; Lin, S.J.; Huang, P.H.; Tarng, D.C. Indoxyl sulfate suppresses endothelial progenitor cell-mediated neovascularization. Kidney Int. 2016, 89, 574–585.
- Iwasaki, Y.; Kazama, J.J.; Yamato, H.; Shimoda, H.; Fukagawa, M. Accumulated uremic toxins attenuate bone mechanical properties in rats with chronic kidney disease. Bone 2013, 57, 477–483.
- Soulage, C.O.; Koppe, L.; Fouque, D. Protein-bound uremic toxins… new targets to prevent insulin resistance and dysmetabolism in patients with chronic kidney disease. J. Ren. Nutr. 2013, 23, 464–466.
- Lau, W.L.; Savoj, J.; Nakata, M.B.; Vaziri, N.D. Altered microbiome in chronic kidney disease: Systemic effects of gut-derived uremic toxins. Clin. Sci. 2018, 132, 509–522.
- Lu, P.-H.; Tai, Y.-C.; Yu, M.-C.; Lin, I.-H.; Kuo, K.-L. Western and complementary alternative medicine treatment of uremic pruritus: A literature review. Tzu Chi Med. J. 2021.
- Kuo, K.-L.; Zhao, J.-F.; Huang, P.-H.; Guo, B.-C.; Tarng, D.-C.; Lee, T.-S. Indoxyl sulfate impairs valsartan-induced neovascularization. Redox Biol. 2020, 30, 101433.
- Vanholder, R.; Glorieux, G.; De Smet, R.; Lameire, N.; European Uremic Toxin Work Group. New insights in uremic toxins. Kidney Int. Suppl. 2003, 63, 1934–1943.
- Castillo-Rodríguez, E.; Pizarro-Sánchez, S.; Sanz, A.; Ramos, A.; Sanchez-Niño, M.; Martin-Cleary, C.; Fernandez-Fernandez, B.; Ortiz, A. Inflammatory Cytokines as Uremic Toxins: “Ni Son Todos Los Que Estan, Ni Estan Todos Los Que Son”. Toxins 2017, 9, 114.
- Vanholder, R.C.; Eloot, S.; Glorieux, G.L. Future Avenues to Decrease Uremic Toxin Concentration. Am. J. Kidney Dis. 2016, 67, 664–676.
- Koppe, L.; Fouque, D.; Soulage, C.O. The Role of Gut Microbiota and Diet on Uremic Retention Solutes Production in the Context of Chronic Kidney Disease. Toxins 2018, 10, 155.
- Popkov, V.A.; Silachev, D.N.; Zalevsky, A.O.; Zorov, D.B.; Plotnikov, E.Y. Mitochondria as a Source and a Target for Uremic Toxins. Int. J. Mol. Sci. 2019, 20, 3094.
- Niwa, T. Indoxyl sulfate is a nephro-vascular toxin. J. Ren. Nutr. 2010, 20, S2–S6.
- Saito, H.; Yoshimura, M.; Saigo, C.; Komori, M.; Nomura, Y.; Yamamoto, Y.; Sagata, M.; Wakida, A.; Chuman, E.; Nishi, K.; et al. Hepatic sulfotransferase as a nephropreventing target by suppression of the uremic toxin indoxyl sulfate accumulation in ischemic acute kidney injury. Toxicol. Sci. 2014, 141, 206–217.
- Sumida, K.; Yamagata, K.; Kovesdy, C.P. Constipation in CKD. Kidney Int. Rep. 2020, 5, 121–134.
- Zhao, Y.; Yu, Y.-B. Intestinal microbiota and chronic constipation. SpringerPlus 2016, 5, 1–8.
- Meijers, B.; Glorieux, G.; Poesen, R.; Bakker, S.J. Nonextracorporeal methods for decreasing uremic solute concentration: A future way to go? Semin. Nephrol. 2014, 34, 228–243.
- Enomoto, A.; Takeda, M.; Taki, K.; Takayama, F.; Noshiro, R.; Niwa, T.; Endou, H. Interactions of human organic anion as well as cation transporters with indoxyl sulfate. Eur. J. Pharmacol. 2003, 466, 13–20.
- Fülöp, T.; Zsom, L.; Tapolyai, M.B.; Molnar, M.Z.; Salim, S.A.; Arany, I.; Hamrahian, M.; Rosivall, L. Peritoneal dialysis: The unique features by compartmental delivery of renal replacement therapy. Med. Hypotheses 2017, 108, 128–132.
- Lameire, N.; Vanholder, R.; De Smet, R. Uremic toxins and peritoneal dialysis. Kidney Int. Suppl. 2001, 78, S292–S297.
- Vanholder, R.; Baurmeister, U.; Brunet, P.; Cohen, G.; Glorieux, G.; Jankowski, J. A bench to bedside view of uremic toxins. J. Am. Soc. Nephrol. 2008, 19, 863–870.
- Lemoine, S.; Pillot, B.; Rognant, N.; Augeul, L.; Rayberin, M.; Varennes, A.; Laville, M.; Ovize, M.; Juillard, L. Postconditioning with cyclosporine a reduces early renal dysfunction by inhibiting mitochondrial permeability transition. Transplantation 2015, 99, 717–723.
- Koyama, K.; Ito, A.; Yamamoto, J.; Nishio, T.; Kajikuri, J.; Dohi, Y.; Ohte, N.; Sano, A.; Nakamura, H.; Kumagai, H.; et al. Randomized controlled trial of the effect of short-term coadministration of methylcobalamin and folate on serum ADMA concentration in patients receiving long-term hemodialysis. Am. J. Kidney Dis. 2010, 55, 1069–1078.
- Evenepoel, P.; Bammens, B.; Verbeke, K.; Vanrenterghem, Y. Acarbose treatment lowers generation and serum concentrations of the protein-bound solute p-cresol: A pilot study. Kidney Int. 2006, 70, 192–198.
- Goicoechea, M.; de Vinuesa, S.G.; Verdalles, U.; Ruiz-Caro, C.; Ampuero, J.; Rincón, A.; Arroyo, D.; Luño, J. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin. J. Am. Soc. Nephrol. 2010, 5, 1388–1393.
- Saigo, C.; Nomura, Y.; Yamamoto, Y.; Sagata, M.; Matsunaga, R.; Jono, H.; Nishi, K.; Saito, H. Meclofenamate elicits a nephropreventing effect in a rat model of ischemic acute kidney injury by suppressing indoxyl sulfate production and restoring renal organic anion transporters. Drug Des. Dev. Ther. 2014, 8, 1073–1082.
- Garibotto, G.; Sofia, A.; Parodi, E.L.; Ansaldo, F.; Bonanni, A.; Picciotto, D.; Signori, A.; Vettore, M.; Tessari, P.; Verzola, D. Effects of Low-Protein, and Supplemented Very Low-Protein Diets, on Muscle Protein Turnover in Patients With CKD. Kidney Int. Rep. 2018, 3, 701–710.
- Huo, X.; Meng, Q.; Wang, C.; Zhu, Y.; Liu, Z.; Ma, X.; Ma, X.; Peng, J.; Sun, H.; Liu, K. Cilastatin protects against imipenem-induced nephrotoxicity via inhibition of renal organic anion transporters (OATs). Acta Pharm. Sin. B 2019, 9, 986–996.
- Asai, M.; Kumakura, S.; Kikuchi, M. Review of the efficacy of AST-120 (KREMEZIN(®)) on renal function in chronic kidney disease patients. Ren. Fail. 2019, 41, 47–56.
- Chen, Y.-C.; Wu, M.-Y.; Hu, P.-J.; Chen, T.-T.; Shen, W.-C.; Chang, W.-C.; Wu, M.-S. Effects and safety of an oral adsorbent on chronic kidney disease progression: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 1718.
- Fatouros, I.G.; Douroudos, I.; Panagoutsos, S.; Pasadakis, P.; Nikolaidis, M.G.; Chatzinikolaou, A.; Sovatzidis, A.; Michailidis, Y.; Jamurtas, A.Z.; Mandalidis, D.; et al. Effects of L-carnitine on oxidative stress responses in patients with renal disease. Med. Sci. Sports Exerc. 2010, 42, 1809–1818.
- Trimarchi, H.; Schiel, A.; Freixas, E.; Díaz, M. Randomized trial of methylcobalamin and folate effects on homocysteine in hemodialysis patients. Nephron 2002, 91, 58–63.
- Marzocco, S.; Dal Piaz, F.; Di Micco, L.; Torraca, S.; Sirico, M.L.; Tartaglia, D.; Autore, G.; Di Iorio, B. Very low protein diet reduces indoxyl sulfate levels in chronic kidney disease. Blood Purif. 2013, 35, 196–201.
- Wang, Y.F. Analysis on the effects of reduced glutathione intervening in microinflammation of uremia patients with maintenance hemodialysis. Chin. J. Front. Med. Sci. 2016, 8, 101–104.
- Sato, E.; Saigusa, D.; Mishima, E.; Uchida, T.; Miura, D.; Morikawa-Ichinose, T.; Kisu, K.; Sekimoto, A.; Saito, R.; Oe, Y.; et al. Impact of the Oral Adsorbent AST-120 on Organ-Specific Accumulation of Uremic Toxins: LC-MS/MS and MS Imaging Techniques. Toxins 2017, 10, 19.
- Sener, G.; Paskaloglu, K.; Satiroglu, H.; Alican, I.; Kaçmaz, A.; Sakarcan, A. L-Carnitine Ameliorates Oxidative Damage due to Chronic Renal Failure in Rats. J. Cardiovasc. Pharmacol. 2004, 43, 698–705.
- Konop, M.; Radkowski, M.; Grochowska, M.; Perlejewski, K.; Samborowska, E.; Ufnal, M. Enalapril decreases rat plasma concentration of TMAO, a gut bacteria-derived cardiovascular marker. Biomarkers 2018, 23, 380–385.
- Armaly, Z.; Artol, S.; Jabbour, A.R.; Saffouri, A.; Habashi, N.; Abd Elkadir, A.; Ghattas, N.; Farah, R.; Kinaneh, S.; Nseir, W. Impact of pretreatment with carnitine and tadalafil on contrast-induced nephropathy in CKD patients. Ren. Fail. 2019, 41, 976–986.
- Ling, X.C.; Kuo, K.-L. Oxidative stress in chronic kidney disease. Ren. Replace. Ther. 2018, 4, 1–9.
- Ceballos-Picot, I.; Witko-Sarsat, V.; Merad-Boudia, M.; Nguyen, A.T.; Thévenin, M.; Jaudon, M.C.; Zingraff, J.; Verger, C.; Jingers, P.; Descamps-Latscha, B. Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic. Biol. Med. 1996, 21, 845–853.
- Zhang, H.; Forman, H.J.; Choi, J. γ-Glutamyl transpeptidase in glutathione biosynthesis. Methods Enzymol. 2005, 401, 468–483.
- Schmitt, B.; Vicenzi, M.; Garrel, C.; Denis, F.M. Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: A comparative crossover study. Redox Biol. 2015, 6, 198–205.
- Snelson, M.; Biruete, A.; McFarlane, C.; Campbell, K. A Renal Clinician’s Guide to the Gut Microbiota. J. Ren. Nutr. 2020, 30, 384–395.
- Di Iorio, B.R.; Rocchetti, M.T.; De Angelis, M.; Cosola, C.; Marzocco, S.; Di Micco, L.; di Bari, I.; Accetturo, M.; Vacca, M.; Gobbetti, M.; et al. Nutritional Therapy Modulates Intestinal Microbiota and Reduces Serum Levels of Total and Free Indoxyl Sulfate and P-Cresyl Sulfate in Chronic Kidney Disease (Medika Study). J. Clin. Med. 2019, 8, 1424.
- Kumar, V.; Yadav, A.K.; Lal, A.; Kumar, V.; Singhal, M.; Billot, L.; Gupta, K.L.; Banerjee, D.; Jha, V. A randomized trial of vitamin D supplementation on vascular function in CKD. J. Am. Soc. Nephrol. 2017, 28, 3100–3108.
- De Vrese, M.; Schrezenmeir, J. Probiotics, prebiotics, and synbiotics. Food Biotechnol. 2008, 111, 1–66.
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555.
- Meirlaen, L.; Levy, E.I.; Vandenplas, Y. Prevention and Management with Pro-, Pre and Synbiotics in Children with Asthma and Allergic Rhinitis: A Narrative Review. Nutrients 2021, 13, 934.
- Nakabayashi, I.; Nakamura, M.; Kawakami, K.; Ohta, T.; Kato, I.; Uchida, K.; Yoshida, M. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: A preliminary study. Nephrol. Dial. Transplant. 2010, 26, 1094–1098.
- Cases, A.; Cigarran-Guldris, S.; Mas, S.; Gonzalez-Parra, E. Vegetable-Based Diets for Chronic Kidney Disease? It is Time to Reconsider. Nutrients 2019, 11, 1263.
- Kandouz, S.; Mohamed, A.S.; Zheng, Y.; Sandeman, S.; Davenport, A. Reduced protein bound uraemic toxins in vegetarian kidney failure patients treated by haemodiafiltration. Hemodial. Int. 2016, 20, 610–617.
- Zhang, Q.-Y.; Jiang, C.-M.; Sun, C.; Tang, T.-F.; Jin, B.; Cao, D.-W.; He, J.-S.; Zhang, M. Hypovitaminosis D is associated with endothelial dysfunction in patients with non-dialysis chronic kidney disease. J. Nephrol. 2015, 28, 471–476.
- Tu, Y.; Sun, W.; Wan, Y.G.; Gao, K.; Liu, H.; Yu, B.Y.; Hu, H.; Huang, Y.R. Dahuang Fuzi Decoction ameliorates tubular epithelial apoptosis and renal damage via inhibiting TGF-beta1-JNK signaling pathway activation in vivo. J. Ethnopharmacol. 2014, 156, 115–124.
- Liu, G.; Zhou, Q.; Tong, X. The clinical application and pharmacological research progress of Dahuang fuzi Decoction. Chin. Arch. Tradit. Chin. Med. 2010, 28, 1848–1851.
- Li, F.; Gong, Q.; Wang, L.; Shi, J. Osthole attenuates focal inflammatory reaction following permanent middle cerebral artery occlusion in rats. Biol. Pharm. Bull. 2012, 35, 1686–1690.
- Luo, L.-N.; Xie, D.Q.; Zhang, X.G.; Jiang, R. Osthole decreases renal ischemia-reperfusion injury by suppressing JAK2/STAT3 signaling activation. Exp. Ther. Med. 2016, 12, 2009–2014.
- Chung, J.H.; Manganiello, V.; Dyck, J.R.B. Resveratrol as a calorie restriction mimetic: Therapeutic implications. Trends Cell Biol. 2012, 22, 546–554.
- Larrosa, M.; Yañéz-Gascón, M.J.; Selma, M.V.; González-Sarrías, A.; Toti, S.; Cerón, J.J.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Effect of a Low Dose of Dietary Resveratrol on Colon Microbiota, Inflammation and Tissue Damage in a DSS-Induced Colitis Rat Model. J. Agric. Food Chem. 2009, 57, 2211–2220.
- Rakici, O.; Kiziltepe, U.; Coskun, B.; Aslamaci, S.; Akar, F. Effects of resveratrol on vascular tone and endothelial function of human saphenous vein and internal mammary artery. Int. J. Cardiol. 2005, 105, 209–215.
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187.
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210-15.
- World Health Organization. Acupuncture: Review and Analysis of Reports on Controlled Clinical Trials; World Health Organization: Geneve, Switzerland, 2002.
- Cabýoglu, M.T.; Ergene, N.; Tan, U. The mechanism of acupuncture and clinical applications. Int. J. Neurosci. 2006, 116, 115–125.
- Yu, J.S.; Ho, C.H.; Wang, H.Y.; Chen, Y.H.; Hsieh, C.L. Acupuncture on Renal Function in Patients with Chronic Kidney Disease: A Single-Blinded, Randomized, Preliminary Controlled Study. J. Altern. Complement. Med. 2017, 23, 624–631.
- Xiong, W.; He, F.F.; You, R.Y.; Xiong, J.; Wang, Y.M.; Zhang, C.; Meng, X.F.; Su, H. Acupuncture Application in Chronic Kidney Disease and its Potential Mechanisms. Am. J. Chin. Med. 2018, 46, 1169–1185.
