Lactococcosis, particularly that caused by Lactococcus garvieae, is a major re-emerging bacterial disease seriously affecting the sustainability of aquaculture industry. Medicinal herbs and plants do not have very much in vitro antagonism and in vivo disease resistance towards lactococcosis agents in aquaculture. Most in vitro studies with herbal extractives were performed against L. garvieae with no strong antibacterial activity, but essential oils, especially those that contain thymol or carvacrol, are more effective.
- lactococcosis
- Lactococcus garvieae
- phytotherapy
- aquaculture
1. Introduction
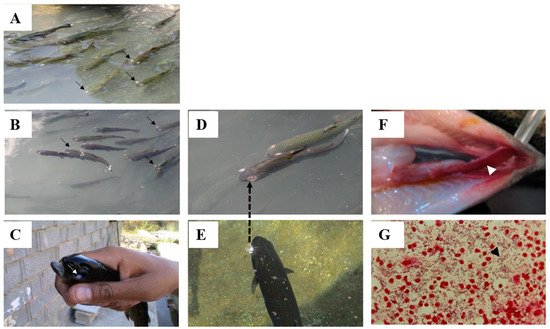
Among the species of Lactococcus genus, L.L. garvieae garvieae has been highlighted as one of the most serious global bacterial pathogens in the aquaculture sector, both in freshwater and marine fish, especially at water temperatures of >15 °C, but L.L. lactis lactis and L. piscium seem to be limited to some highly valuable aquaculture species, such as salmonids and sturgeons, at various water temperatures [1][2][3][11,22,23]. Due to the widespread sources of the bacterial agents and disease spreading, as well as the heterogenicity of the bacterial stains implicated in the disease outbreaks, both vaccination and chemotherapy require more attention in future. The application of co-friendly environmental substances, such as medicinal herbs and probiotics, are nowadays a potential best alternative to antibiotic therapy and an immune enhancer against such bacterial diseases.
2. Diseases Caused by Lactococcus Members in Aquaculture
2.1. Disease Caused by L. garvieae
2.1. Disease Caused by L. garvieae
2.2. Diseases Caused by Other Species of Lactococcus Genus
L.
2.2. Diseases Caused by Other Species of Lactococcus Genus
L. lactis lactis strains are genetically classified into four subspecies of lactis, cremoris, tractae, and hordniae [20][13]. It is not a common veterinary pathogen, although it can cause cattle mastitis and be involved in septic arthritis of the neonatal calf. For example, several variants of L.L. lactis lactis have been associated with bovine mastitis [21][79]. In humans, it has been reported as a cause of endocarditis, arthritis, and septicemia in patients, although this requires more clarification [22][23][24][25][26][80,81,82,83,84].
Up to date, there are only four reports of lactococcosis by L.L. lactis lactis in an aquatic organisms. The first report was an outbreak of white tail disease in cultured giant freshwater prawn (Macrobrachium rosenbergii) in Taiwan [27][85]. The affected prawns were cloudy and whitish in the muscles, showing remarkable edema and necrosis and inflammation in the muscles and hepatopancreas. In subsequent report by Chen et al. [3][23] L.L. lactis lactis subsp. Lactis was isolated from affected hybrid sturgeon, Bester (Huso huso x Acipenser ruthenus) with signs of anorexia, pale body color, reddish spots on the abdomen, enteritis, enlarged abdomen, rapid respiration rate ascites, and 70%–100% mortality. Microscopically, the affected sturgeons demonstrated extensive haemorrhagic multifocal necrotic foci of spleen and liver with degeneration of hepatic cells, lipid droplets and glycogen granules, necrosis and renal tubule epithelial swelling and hydropic degeneration in kidney, skin ulcers deep in underling muscles, appearance of present of immunocompetent cells in the stomach, and small focus on tips of gills and on the myocardium [3][23]. No histopathological changes were, however seen in the eyeball, cerebrum and meninges of affected fish. The third report was from silver carp (Hypophthalmichthys molitrix) with extensive skin lesions near the caudal peduncle and musculoskeletal lesion in the USA [28][86]. The fourth outbreak of infection by L.L. lactis lactis has been reported as the cause of endocarditis valvularis, parientalis thromboticans in mature allis shad (Alosa alosa) in Europe in 2018 that could be associated with the stressors, such as capturing, transport, breeding, and low oxygen level [29][87]. Although, in some cases the disease was reproduced experimentally, the mechanisms of pathogenesis by L.L. lactis lactis in aquatic animals warranted future research works.
3. Phytotherapy of Lactococcosis in Aquaculture
3.1. In Vitro Studies
| Origin/ Source of L. garvieae |
Plant | Extractive | Major Compounds | MIC | MBC | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rainbow trout | Camellia sinensis (green tea-leaves) |
Methanolic extract | Unknown | 800 | rosenbergii) | Eichhornia crassipes (water hyacinth-leaves) | Hot-water extract | 1 g kg diet−1, 12 days 2 g kg diet−1, 12 days1100 |
3 g kg dietAkbary, 2014 |
|||
| −1 | , 12 days | ↑57.3 | ↑128.6 ↑171.4 |
Chang et al., 2013 | Rainbow trout | Thymus vulgaris (thyme), Origanum vulgare (oregano) and Eucalyptus sp. | ||||||
| Giant freshwater prawn (M. rosenbergii) | Mix-oil® (essential oils from leaves) | Citraconic anhydride, 1, 8-cineole, thymol | Eichhornia crassipes (leaves) | Powder6.25 | 12.5 | Amiri et al., 2020 | ||||||
| 20 g kg diet | −1 | , 120 days | ↑44.3 | Chang et al., 2016 | Unknown | Glycyrrhiza glabra L. (black sugar-root) | n-hexane extract | |||||
| Unknown | Unknown | 5630 | Asan-Ozusaglam et al., 2014 | |||||||||
| Hot-water extract | 20 g kg diet−1, 120 days | ↑89.0 | Unknown | Glycyrrhiza glabra L. (root) | Dichloromethane extract | Unknown | Unknown | 1410 | Asan-Ozusaglam et al., 2014 | |||
| Aqueous extract 1 | 2 g kg diet−1, 120 days | ↑89.0 | Type culture collection | Lavandula angustifolia (lavender) | Essential oil | |||||||
| Dreg of aqueous extract 1Unknown | 500 | Unknown | Baba, 2020 | |||||||||
| 18 g kg diet | −1 | , 120 days | ↑77.7 | Type culture collection | Eugenia caryophyllus | |||||||
| Giant freshwater prawn (M. rosenbergii) | Essential oil | Unknown | Musa acuminate (banana-peel) | Aqueous extract | 1 g kg diet250 | Unknown | Baba, 2020 | |||||
| −1 | , 120 days | 3 g kg diet−1, 120 days 6 g kg diet−1, 120 days |
↑200 ↑300 |
Rainbow trout | Mentha piperitae (pepper mint) | Essential oil | Unknown | 500 | Unknown | Baba, 2020 | ||
| ↑467 | Rattanavichai et al., 2015 | |||||||||||
| Giant freshwater prawn (M. rosenbergii) |
Morinda cutrifolia (noni) | Aqueous extract | 0.6 g kg diet−1, 21 days 3 g kg diet−1, 21 days 6 g kg diet−1, 21 days |
↑250 ↑50.4 NS |
Halim et al., 2017 | Rainbow trout | Rosmarinus officinalis (rosemary) | Essential oil | Unknown | |||
| Nile tilapia | 500 | (Oreochromis niloticus) | Unknown | Baba, 2020 | ||||||||
| Argania spinosa (argan-seeds) | Oil | 5 mL kg diet−1, 45 days 10 mL kg diet−1, 45 days 20 mL kg diet−1, 45 days |
↑66.7 ↑91.7 ↑86.1 |
Baba et al., 2017 | Rainbow trout | Cinnamomum zeylanicum (cinnamon) | Essential oil | Unknown | 250 | Unknown | Baba, 2020 | |
| Rainbow trout (Oncorhynchus mykiss) |
Lentinula edodes (Shiitake mushroom) | Aqueous extract | 10 g kg diet−1, 45 days 20 g kg diet−1, 45 days |
↑79.0 ↑109.7 |
Baba et al., 2015 | Rainbow trout | Nigella sativa (black cumin) | Essential oil | Unknown | 250 | Unknown | Baba, 2020 |
| Rainbow trout (O. mykiss) |
Pleurotus ostreatus (oyster mushroom) | Aqueous extract | 10 g kg diet−1, 42 days 20 g kg diet−1, 42 days |
↑40.0 ↑60.1 |
Uluköy et al., 2016 | Strain O41 | Lavandula officinalis (true lavender-flowers) | Ethanolic extract | Essential oil (linalyl acetate), tannins, coumarins, flavonoids, and phytosterols |
4200 | 8400 | Bulfon et al., 2014 |
| Rainbow trout (O. mykiss) |
Usnea barbata (beard lichen) | Methanolic extract | 230 mg kg fish−1 (a) 460 mg kg fish−1 (a) 690 mg kg fish−1 (a) |
↑62.4 ↑45.3 NS |
Bilen et al., 2019 | Strain O41 | Melissa officinalis (lemon balm-leaves) | Ethanolic extract | Rosmarinic acid, essential oil (citral, | |||
| Three spotted tilapia (Oreochromis andersonii) | Capsaicincitronellal, β-caryophyllen), caffeic acid, and chlorogenic acidderivatives | 8400 | 33,600 | Bulfon et al., 2014 | ||||||||
| Isolated compound | 1.97 mg kg fish−1 (b) | 80% survival vs. 0% survival in control | Strain O41 | Ocimum basilicum (sweet basil-flowering plant) | Ethanolic extract | Essential oil (linalool, estragol, camphor, eugenol, ocimene, cineol, sesquiterpenes), tannins, favonoids, caffeic acid, and esculoside | 16,800 | Unknown | Bulfon et al., 2014 | |||
| Strain O41 | Origanum vulgare (oregano-inflorescence) | Ethanolic extract | Carvacrol, thymol, γ-terpinene, p-cymene, limonene, linolool, and borneol | 4200 | 33,600 | Bulfon et al., 2014 | ||||||
| Ndashe et al., 2020 | Strain O41 | Orthosiphon stamineus (Java tea-leaves) | Ethanolic extract | Essential oil (sesquiterpenes), flavones, triterpenoid, saponins, vitamins, and organic salts | 33,600 | Unknown | Bulfon et al., 2014 | |||||
| Strain O41 | Rosmarinus officinalis (leaves) | Ethanolic extract | Essential oil (eucalyptol, α-pinene, camphor, borneol), flavonoids, rosmarinic acid, and terpenes | 8400 | Unknown | Bulfon et al., 2014 | ||||||
| Strain O41 | Salvia officinalis (sage-leaves) | Ethanolic extract | Essential oil (thujone, monoterpenes, and sesquiterpenes), tannins, bitter substances, and flavonoids | 4200 | 33,600 | Bulfon et al., 2014 | ||||||
| Strain O41 | Thymus vulgaris (leaves) | Ethanolic extract | Essential oil (thymol, carvacrol, p-cimol, and terpinene), tannins, flavonoids and triterpenes | Unknown | Unknown | Bulfon et al., 2014 | ||||||
| Strain O41 | Vaccinium vitis-idaea (lingonberry-leaves) | Ethanolic extract | Phenolic glycosides (arbutin and hydroquinone), tannins, flavonoids (iperoside, avicularin, isoquercitrin), terpenic acids (ursolic and oleanolic acids), organic acids, and mineral salts | 4200 | Unknown | Bulfon et al., 2014 | ||||||
| Rainbow trout | Glycyrrhiza glabra L. (root) | Ethanolic extract | Unknown | 920 | Unknown | Fereidouni et al., 2013 | ||||||
| Rainbow trout | Peganum harmala (wild rue-seed) | Methanolic extrac | Unknown | 105 | Unknown | Fereidouni et al., 2013 | ||||||
| Rainbow trout | Trachyspermum copticum (carum ajowan-seed) | Ethanolic extract | Unknown | 453 | Unknown | Fereidouni et al., 2013 | ||||||
| Rainbow trout | Myrtus communis (myrtle-leaves) | Essential oil | Unknown | 672 | Unknown | Fereidouni et al., 2013 | ||||||
| Rainbow trout | Juglans regia (English walnut-leaves) | Ethanolic extract | Unknown | 510 | Unknown | Fereidouni et al., 2013 | ||||||
| Rainbow trout | Quercus branti Lindley (Brant’s oak-seed) | Ethanolic extract | Unknown | 978 | Unknown | Fereidouni et al., 2013 | ||||||
| Rainbow trout | Tanacetum parthenium (feverfew-leaves) | Essential oil | Unknown | 824 | Unknown | Fereidouni et al., 2013 | ||||||
| Rainbow trout | Satureja bachtiarica Bung. (savory-leaves) | Essential oil | Unknown | 126 | Unknown | Fereidouni et al., 2013 | ||||||
| Rainbow trout | Glycyrrhiza glabra L.(root) | Ethanolic extract | Glycyrrhizinic acid | >1000 | >1000 | Goudarzi et al., 2011 | ||||||
| Rainbow trout | Satureja bachtiarica Bung. (aerial parts) | Essential oil | Phenols: Carvacrol, thymol | 8 | 16 | Goudarzi et al., 2011 | ||||||
| Rainbow trout | Satureja bachtiarica Bung. (aerial parts) | Ethanolic extract | Phenols: Carvacrol, thymol | >1000 | >1000 | Goudarzi et al., 2011 | ||||||
| Rainbow trout | Punica granatum (pomegranate-flowers) | Ethanolic extract | Polyphenols: pomegranatate | >1000 | >1000 | Goudarzi et al., 2011 | ||||||
| Essential oil | D-carvacrol, limonene, dill apiole, E-dihydrocarvone, Z-dihydrocarvone | 62.4 | 125 | Mahmoodi et al., 2012 | ||||||||
| L. garvieae GQ850376 | ||||||||||||
| Rainbow trout (O. mykiss) |
Origanum onites (oregano) | Essential oil | 0.125 mL kg diet−1, 56 days 1.5 mL kg diet−1, 56 days 2.5 mL kg diet−1, 56 days 3.0 mL kg diet−1, 56 days |
↑54 ↑92 ↑84 |
Rainbow trout | Quercus branti Lindley (seed/flour) | Ethanolic extract | Tannins | >1000 | >1000 | Goudarzi et al., 2011 | |
| Rainbow trout | Echinophora platyloba DC. (prickly parsnip-aerial parts) | Essential oil | Monoterpenes: trans-β-ocimene | >1000 | >1000 | Goudarzi et al., 2011 | ||||||
| Rainbow trout | Echinophora platyloba DC. (aerial parts) | Ethanolic extract | Monoterpenes: trans-β-ocimene | >1000 | >1000 | Goudarzi et al., 2011 | ||||||
| Rainbow trout | Heracleum lasiopetalum Boiss. (fruits) | Ethanolic extract | Sesquiterpene hydrocarbons: Germacrene-D | >1000 | >1000 | Goudarzi et al., 2011 | ||||||
| Rainbow trout |
Kelussia odoratissima Mozaff. (wild celery-leaves) | Ethanolic extract | Z-ligustilide | >1000 | >1000 | Goudarzi et al., 2011 | ||||||
| Rainbow trout | Stachys lavandulifolia Vahl (wood betony-flowers) | Ethanolic extract | Sabinene, α-pinene, β-myrcene | >1000 | >1000 | Goudarzi et al., 2011 | ||||||
| No mortality | Diler et al. 2017 | |||||||||||
| Nile tilapia (Oreochromis niloticus) |
Syzygium aromaticum (clove-buds) | Essential oil | 5 mL kg diet−1, 5 days 10 mL kg diet−1, 5 days 20 mL kg diet−1, 5 days 30 mL kg diet−1, 5 days |
↑40 ↑70 ↑80 No mortality |
Rattanachaikunsopon et al., 2009 | |||||||
| Mullet (Mugil cephalus) | * TCM | Aqueous extract of the powder | 5 g kg fish−1, 28 days 10 g kg fish−1, 28 days |
Rainbow trout | Thymus daenensis Celak. (common thyme-aerial part/inflorescence) | Ethanolic extract | Phenols: thymol, carvacrol | >1000 | >1000 | Goudarzi et al., 2011 | ||
| Rainbow trout | Thymus daenensis Celak. (Aerial part/inflorescence) | Essential oil | Phenols: thymol, carvacrol | 8 | 16 | Goudarzi et al., 2011 | ||||||
| Rainbow trout | Myrtus communis (leaves) | Ethanolic extract | α-pinene, 1,8-cineole, myrtenyl acetate | >250 | >500 | Goudarzi et al., 2011 | ||||||
| Rainbow trout | Myrtus communis (leaves) | Essential oil | α-pinene, 1,8-cineole, myrtenyl acetate | >1000 | >1000 | Goudarzi et al., 2011 | ||||||
| Rainbow trout | Thymbra spicata (spiked thyme-aerial part/inflorescence) | Essential oil | Phenols: thymol, carvacrol | 8 | 16 | Goudarzi et al., 2011 | ||||||
| Rainbow trout | Bunium persicum (Boiss.) K.- Pol. (black caraway-fruits) |
Essential oil | γ-terpinen-7-al, cuminaldehyde, γ terpinene | 8 | 16 | Goudarzi et al., 2011 | ||||||
| 20 g kg fish | −1 | , 28 days | NS | Rainbow trout | Teucrium polium (felty germander-aerial parts) | Essential oil | α-pinene, linalool | >1000 | >1000 | Goudarzi et al., 2011 | ||
| Rainbow trout | Alhagi maurorum (camelthorn-aerial parts) | Essential oil | Alhagidin, alhagitin, quercetin, catechin | >1000 | >1000 | Goudarzi et al., 2011 | ||||||
| ↑230.8 | ↑184.6 | Choi et al., 2014 | ||||||||||
| Rainbow trout (O. mykiss) |
Citrus paradisi (grapefruit), Citrus reticulata (tangerine), Citrus aurantium ssp. bergamia (bergamot), Citrus sinensis (sweet orange) | Biocitro ® (blend of these extracts) | 0.75 g kg diet−1, 28 days | Rainbow trout | Zataria multifora Boiss. (Shirazi thyme) | Essential oil | Phenols: thymol, carvacrol | 4 | 8 | Goudarzi et al., 2011 | ||
| ↑120 | Olive flounder (P. olivaceus) | Zingiber officinale (ginger) | Essential oil | 2000 | 2000 | Hossain et al., 2019 | ||||||
| Mora-Sánchez et al., 2020 | L. garvieae GQ850376 | Eucalyptus globulus (southern blue gum-aerial parts) | Essential oil | 1,8-eucalypol, pinene, terpineol acetae, globulol | 250 | 250 | Mahmoodi et al., 2012 | |||||
| L. garvieae GQ850376 | Eucalyptus globulus (aerial parts) | Methanolic extract | 1,8-eucalypol, pinene, terpineol acetae, globulol | 500 | 500 | Mahmoodi et al., 2012 | ||||||
| L. garvieae GQ850376 | Zataria multiflora (aerial parts) | Essential oil | phenolic monoterpene, Carvacrol, alpha-pinene | 7.8 | 15.6 | Mahmoodi et al., 2012 | ||||||
| L. garvieae GQ850376 | Zataria multiflora (aerial parts) | Methanolic extract | phenolic monoterpene Carvacrol, alpha-pinene | 15.6 | 15.6 | Mahmoodi et al., 2012 | ||||||
| L. garvieae GQ850376 | Anethum graveolens (dill-seed) | Anethum graveolens (seed) | Methanolic extract | D-carvacrol, limonene, dill apiole, E-dihydrocarvone, Z-dihydrocarvone | 125 | 125 | Mahmoodi et al., 2012 | |||||
| L. garvieae GQ850376 | Rosmarinus officinalis | Essential oil | 1,8-cineole, alpha-pinene, toluene | 15.6 | 31.2 | Mahmoodi et al., 2012 | ||||||
| L. garvieae GQ850376 | Rosmarinus officinalis | Methanolic extract | 1,8-cineole, alpha-pinene, toluene | 31.2 | 31.2 | Mahmoodi et al., 2012 | ||||||
| Rainbow trout | Citrus paradisi (grapefruit), Citrus reticulata (tangerine), Citrus aurantium ssp. bergamia (bergamot), Citrus sinensis (sweet orange) | Biocitro ® (blend of citrus extracts) | Ascorbic acid, citrus bioflavonoids (hesperidin, naringin, quercetin, rutin) and organic acids | 2.0 | Unknown | Mora-Sánchez et al., 2020 | ||||||
| Tilapia (O. andersonii) | Capsicum annum (Chili pepper) | Methanolic extract (capsaicin) | Unknown | Unknown | 196.7 | Ndashe et al., 2020 | ||||||
| Olive flounder | Citrus aurantifolia (key lime-peel) | Essential oil | Limonene, γ-terpinene, β-pinene | 0.125% (v/v) | 1% (v/v) | Pathirana et al., 2018 | ||||||
| Olive flounder | Limonene | Commercial trans-limonene (>99%) | Limonene | 0.031% (v/v) | 0.025% (v/v) | Pathirana et al., 2018 | ||||||
| Olive flounder | Syzygium aromaticum (clove-buds) | Essential oil | Eugenol, β-caryophyllene, α-humulen, eugenyl-acetate | 0.5% (v/v) | 1% (v/v) | Pathirana et al., 2019a | ||||||
| Olive flounder | Commercial eugenol (>99%) | Isolated compound eugenol |
Eugenol | 1% (v/v) | 1% (v/v) | Pathirana et al., 2019a | ||||||
| Olive flounder | Cinnamomum zeylanicum | Essential oil | Cinnamaldehyde, eugenol, β-Caryophyllene |
0.015% (v/v) | 0.031% (v/v) | Pathirana et al., 2019b | ||||||
| Olive flounder | Commercial trans-cinnamaldehyde (>99%) (Sigma-Aldrich) | cinnamaldehyde | Cinnamaldehyde | 0.003% (v/v) | 0.015% (v/v) | Pathirana et al., 2019b | ||||||
| Rainbow trout | Argania spinose L. (argan-oil) | Essential oil | Oleic acid, linoleic acid, palmitic acid, stearic acid | 250 | Unknown | Öntas et al., 2016 | ||||||
| Rainbow trout | Citrus limon L. (lemon-peel) | Essential oil | Limonene, γ-terpinene, β-pinene, α-terpineol, myrecene and terpinolene | 500 | Unknown | Öntas et al., 2016 | ||||||
| Strain ATCC43921 | Cinnamomum verum (cinnamon-bark) | Essential oil | Unknown | 120 | Unknown | Rattanachaikunsopon et al., 2009 | ||||||
| Strain ATCC43921 | Ocimum sanctum (holy basil-leaves) | Essential oil | Unknown | 240 | Unknown | Rattanachaikunsopon et al., 2009 | ||||||
| Strain ATCC43921 | Zingiber officinale (roots) | Essential oil | Unknown | 120 | Unknown | Rattanachaikunsopon et al. 2009 | ||||||
| Strain ATCC43921 | Syzygium aromaticum (flower buds) | Essential oil | Unknown | 30 | Unknown | Rattanachaikunsopon et al., 2009 | ||||||
| Rainbow trout | Zataria multiflora (aerial parts) | Essential oil | Carvacrol, benzene and phenol | 0.12 | 0.12 | Soltani et al., 2014 | ||||||
| Rainbow trout | Allium sativum (garlic-edible parts) | Essential oil | trisulfide, di-2-propenyl, disulfide, di-2-propenyl and trisulfide, methyl 2-propenyl | 0.5 | 1 | Soltani et al., 2014 | ||||||
| Rainbow trout | Cinnamomum zeylanicum (bark) | Essential oil | cinnamic aldehyde, linalool, ortho methoxy cinnamic aldehyde and 1,8-cineole | 0.5 | 0.5 | Soltani et al., 2014 | ||||||
| S. quinqueradiata | Chloramphenicol | 0.8 a | 1.6 b | Maki et al., 2008 | ||||||||
| S. quinqueradiata | Ciprofloxacin | 1.6 a | 3.13 b | Maki et al., 2008 | ||||||||
| S. quinqueradiata | Erythromycin | 0.1 a | 800 b | Maki et al., 2008 | ||||||||
| S. quinqueradiata | Enoxacin | 6.25 a | 12.5 b | Maki et al., 2008 | ||||||||
| S. quinqueradiata | Florfenicol | 1.6 a | 1.6 b | Maki et al., 2008 | ||||||||
| S. quinqueradiata | Floroxacin | 12.5 a | 12.5 b | Maki et al., 2008 | ||||||||
| S. quinqueradiata | Kanamycin | 25 a | 50 b | Maki et al., 2008 | ||||||||
| S. quinqueradiata | Lincomycin | 25 a | 800 b | Maki et al., 2008 | ||||||||
| S. quinqueradiata | Norfloxacin | 6.25 a | 12.5 b | Maki et al., 2008 | ||||||||
| S. quinqueradiata | Oxolinic acid | 400 a | 800 b | Maki et al., 2008 | ||||||||
| S. quinqueradiata | Orbifloxacin | 1.6 a | 1.6 b | Maki et al., 2008 | ||||||||
| S. quinqueradiata | Ofloxacin | 3.13 a | 6.25 b | Maki et al., 2008 | ||||||||
| S. quinqueradiata | Penzylpenicillin | 0.8 a | 1.6 b | Maki et al., 2008 | ||||||||
| S. quinqueradiata | Streptomycin | 25 a | 50 b | Maki et al., 2008 | ||||||||
| S. quinqueradiata | Tetracycline | 12.5 a | 400 b | Maki et al., 2008 |
| Host | Plant | Extractive | Dosage/Duration | Survival Increase Compared to Control (%) 2 | Reference |
|---|---|---|---|---|---|
| Giant freshwater prawn (Macrobrachium |
3.2. In Vivo Studies
3.2. In Vivo Studies
All in vivo studies were related to survival against L.L. garvieae garvieae infection, and, in most cases, the extractives of medicinal herbs and plants were added to the diets for various periods before the treated fish being challenged with L.L. garvieae garvieae infection. Overall, the essential oils that showed the best in vitro antibacterial activity against L.L. garvieae garvieae (Table 1) were not tested for the in vivo bioassays yet. The extractives tested under in vivo conditions presented moderate in vitro antibacterial activity against this bacterium or even were not tested in vitro. However, the dietary supplementation with all tested extractives reduced mortality of infected animals (Table 2), probably because they improved immune parameters before challenging the treated fish with LL. garvieae. garvieae. A 12-day feeding giant freshwater prawn (Macrobrachium rosenbergii) with hot-water extract of water hyacinth (Eichhornia crassipes) leaves at 1, 2, and 3 g kg−1 diet induced significantly higher survival rate after challenge with L.L. garvieae garvieae infection, but higher disease resistance was seen in the prawn treated with higher concentration of the extract [30][108]. In addition, the treated animals exhibited an enhancement in the immune responses including respiratory burst, phenoloxidase activity, superoxide dismutase activity, glutathione peroxidase, total hemocyte value, differential hemocyte count, transglutaminase activity, and phagocytic activity towards LL. garvtieae. garvtieae. In the subsequent research work by Chang and Cheng [31][109], dietary addition of three tested water hyacinth extracts (Table 2) for 120 days increased survival and immune parameters, i.e., total hemocyte count, semi-granular and granular cells counts of giant freshwater prawn while phenoloxidase activity, respiratory bursts of hemocytes were not observed only with dietary addition of powder of this plant to the diet.
