Human milk promotes optimum growth, development, and health of the infant; however the mechanisms that govern both the variation of composition and the pathways by which it delivers benefits to the infant are not well understood. Increasingly maternal and environmental factors are being associated with milk composition. A recent systematic review indicated maternal adiposity was related to HM lactose and fat concentrations.
- lactation
- breastfeeding
- human milk
- milk composition
- breast
- infant feeding
1. Introduction
Breastfeeding is a major determinant of infant short- and long-term health, preventing acute infections and programming lower risk for chronic disease and is therefore considered to be a major public health focus [1]. Despite the wealth of information on the benefits to both the mother and the infant, global breastfeeding rates remain low, averaging only 41% [2]. To identify and address the issues faced by the breastfeeding dyad a foundational knowledge of the ‘norm’ is required. In the haste to provide support and solutions, an understanding of the basic science is frequently neglected in favour of the implementation of interventional trials that promise advances in care but are themselves based on an incomplete foundational understanding. The dearth of research into the fundamentals of human lactation over the latter part of the last century, combined with the lack of translation of the limited research actually performed into the medical model, has left clinicians little scope and tools to objectively assess breastfeeding problems. Evidence-based care, established from an understanding of the basic principles of lactation and mammary gland function is imperative to improve breastfeeding rates and subsequently the health and wellbeing of breastfeeding women, infants’, and their families.
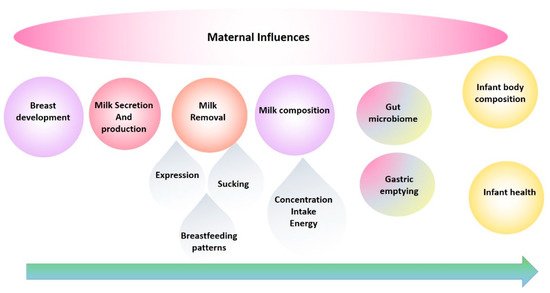
The evolution of our research program has been in direct response to the need to fill the translation gap and has been a journey from basic research into the anatomy, function of the breast and milk composition to multidimensional translational research elucidating mechanisms by which breastfeeding confers a multitude of benefits. These studies have provided the foundation for the development of successful interventions for those mothers experiencing lactation difficulties and have informed and reassured those women, particularly first-time mothers, that they are ‘normal’. This road has not been smooth, with findings that have disrupted the field requiring the reassessment of conventional dogma and change of deep-seated beliefs. Importantly, the rewards of change have been tangible and impactful for mothers and their babies. This review traces the main focal points of our research programme over the last 25 years and takes a biological systems approach by tracking breast milk from the mother (the breast) to the infant ( Figure 1 ) [3] and aims to highlight and document these findings in the broader context of mammary gland function and the breastfeeding dyad, in order to provide a holistic platform of knowledge from which others may continue to work from in their pursuit to define better health outcomes for the next generation [4].
2. Breast Physiology
Typically, breast growth is obvious in pregnancy due to proliferation of glandular tissue and the differentiation lactocytes to produce milk [5][6][10,77]. Breast size however is not indicative of milk production although women with smaller breasts are more likely to have smaller storage capacities and therefore feed more frequently than those women with larger breasts [6][77].
Increasing evidence also suggests that pregnancy complications such as gestational diabetes mellitus, preeclampsia, gestational hypertension [7][78], and fetal growth restriction [8][79] are associated with shorter durations of breastfeeding. While caesarean section has been associated with delayed initiation of breastfeeding, in those women that breastfeed secretory activation [9][38] and any breastfeeding at 6 months appears to not differ between caesarean section and vaginal delivery [10][80]. In addition, women experiencing postpartum haemorrhage, including those that receive a blood transfusion in hospital, also show reduced breastfeeding at discharge, irrespective of haemoglobin concentration pre-transfusion and persistence of anaemia post transfusion [11][12][81,82].
Mastitis or inflammatory lactating breast conditions often result in increased permeability of the alveolus, evidenced by increased human milk (HM) sodium, chloride, lactoferrin, serum albumin concentrations and decreased HM lactose and glucose as well as increased 24-h excretion of lactose, blood, and milk C-reactive protein [13][14][15][83,84,85]. Furthermore, we have observed greater numbers of immune cells and expression of immune proteins such as granzyme B [16][86] in the milk of women with mastitis. Many of the changes in milk composition seen with mastitis are observed with perceived and measured low milk supply supporting maternal reports of reduced milk supply with mastitic episodes [13][83].
Blocked ducts are also commonly experienced during lactation and can be associated with engorgement and inflammatory symptoms [17][87], yet little is understood about the causes and effective treatments [18][88]. Whilst a blocked duct may resolve in 24 h with increased milk removal and massage, non-resolution of these breast masses should cause concern and initiate imaging investigations such as ultrasound to exclude other causes such as fibroadenomas, cysts, lymph nodes and malignancy [19][89].
3. Human Milk Microbiome
The development of the gut microbiome in the first years of life has been strongly associated with immune and metabolic outcomes in large human cohort studies, and in interventional animal models [20][264]. Importantly, HM shapes the infant microbiome through direct transfer of bacteria as well as bioactive components such as HMOs, antimicrobial proteins, and short chain fatty acids (SCFAs) [21][265]. The human milk microbiome (HMM) is therefore of great interest as a target for developmental programming of health. However, while the composition of the HMM has been extensively characterised ([21][265]), little is known about the origins of this community or the host-microbiome interactions at the breast. Host-microbe and microbe-microbe interactions in the lactating mammary gland are likely to be highly complex. Inter-kingdom interactions between human cells, bacteria, fungi, and viruses, as well as interactions involving host-derived and microbe-derived bioactives likely influence mammary gland and infant health ( Figure 214 ). Indeed, it is not even known whether the lactating mammary gland hosts a permanent resident microbiome, or whether bacteria are bought in from exogenous locations (such as the maternal gut or infant oral cavity) and survive temporarily before being swept out of the breast via a ME ( Figure 214 ). Stinson et al. have described these two possibilities as the “mucosal interface model” and the “constant influx model”, respectively [21][265]. Regardless of whether the bacteria detected in milk are permanent residents or mere “tourists”, numerous HMM taxa have been shown to be vertically transferred from mother to infant via milk [22][23][24][25][266,267,268,269]. Factors that shape HMM composition, such as maternal diet, may thereby influence infant colonisation dynamics, with implications for infant health. The potential for maternal diet during lactation to influence infant gut microbiome dynamics has been reviewed extensively by Sindi et al. [26][270]. This evidence, from observational human studies and animal models, paves the way for future intervention studies to assess the impact of maternal diet on infant microbial development.
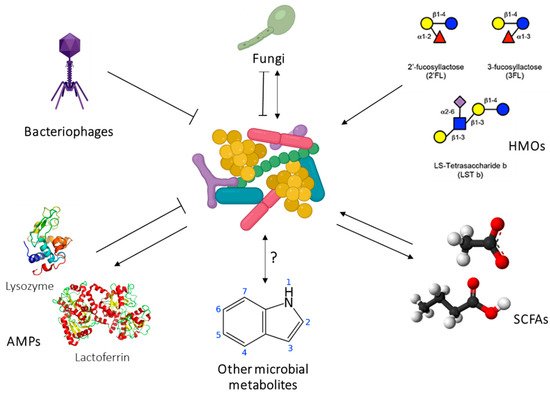
In addition to live bacteria, HM also contains the products of bacterial metabolism, such as SCFAs. These immunomodulatory metabolites SCFAs (formate, acetate, propionate, butyrate, and valerate) are the end products of bacterial fermentation of fibre in the gut and are transported systemically around the body, including to the lactating mammary gland. They have been shown to elicit a broad range of immunological effects, including promotion of regulatory T cell responses and immune tolerance, synthesis of dendritic cell precursors, and epithelial barrier integrity in the gut [29][30][31][273,274,275]. HM therefore has a two-fold influence on infant health: by directly seeding the infant microbiome with human milk bacteria, and by exposing the infant to bacterial metabolites formed in the maternal gut. Emerging evidence suggests that SCFAs may protect infants from developing atopic disease [29][32][273,276]. Our research has shown that milk from atopic mothers contains a significantly reduced concentration of SCFAs compared to that of healthy mothers [33][277]. This finding may in part explain why breastfeeding does not protect against atopy if the mother herself is atopic [34][35][278,279]. HM SCFAs have also been associated with infant BC [36][280]. They are therefore of interest for both infant growth and infant immune development. Given that maternal SCFA levels may be modulated by diet, these bacterially derived metabolites represent an exciting opportunity for intervention to optimise infant health.
Our group has established methodologies for studying the HMM from collection to analysis. We demonstrated that milk expressed using an electric breast pump does not differ in its bacterial composition to milk expressed by hand [37][281]. This finding is reassuring for those designing HMM studies. We also assessed four commercial DNA extraction kits for their ability to extract DNA from HM [38][282]. We found that two of the kits could not reliably extract DNA from HM. Of the two remaining kits, a similar bacterial DNA profile was extracted, but one kit co-extracted a high level of contaminants. Such inter-kit variability may help to explain some of the variation seen in HMM composition between studies. A significant challenge in working with HM is the fat fraction, which interferes with DNA extraction. This fraction is therefore routinely discarded prior to DNA extraction in HMM studies. We demonstrated that this fraction contains bacterial DNA, suggesting that bacteria may be trapped in the lipid layer by milk fat globule membranes [39][40][283,284]. However, reassuringly, the fat fraction did not differ in bacterial composition to the cell pellet, suggesting that discarding the fat fraction prior to extraction would not alter the composition of bacteria detected downstream. This is particularly important, as we have shown that inclusion of the fat fraction reduces DNA extraction efficiency by ~40% [39][283]. By focusing on robust methodologies for reproducible data, we have raised the standards of the field.
4. Donor Human Milk
In past decades there has been a resurgence in the establishment of donor milk banks in an effort to provide HM for vulnerable infants [41][285]. Donor milk has been shown to have positive effects of infant mortality and morbidity [42][286] whilst being economically cost effective [43][287]. We have been active in the formulation of best practice in milk banking which varies according to geography and resources [44][288] as well as investigating pasteurization methods. Thermal pasteurization of milk is almost universal in milk banks however in the process of eliminating bacteria and most viruses [45][46][289,290] bioactivity is often dramatically reduced [47][291]. Reduction in the loss of bioactivity can be altered by reduction in pasteurization temperature [48][292], combinations of time and temperature, as well as other technologies [47][49][291,293]. Our group has pioneered UV-C treatment [50][294] in an effort to preserve the bioactivity of protective components in milk. It shows great promise in eliminating bacteria [51][295] including cytomegalovirus [52][296] not at the expense of immune protein activity [53][297]. More recently we investigated the heat stable enterotoxin produced by the potential pathogen S. aureus. S. aureus enterotoxins are linked to gastritis and necrotizing enterocolitis. Spike in experiments (S. aureus, and enterotoxin) at various storage temperatures and times confirmed a rapid decline in both the bacteria and enterotoxin in raw and UV-C treated milk providing more evidence of promise for this method of processing donor milk [54][55][298,299].
