Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 3 by Camila Xu.
The thermally conductive polymer is usually categorized into the intrinsic thermally conductive polymers and the thermally conductive polymer composites.
- thermal management material
- thermal conductivity
1. Intrinsic Thermally Conductive Polymers
On the basis of the preparation process, the thermally conductive polymer is usually categorized into the intrinsic thermally conductive polymers and the thermally conductive polymer composites [1][10]. The thermal conductivity of solid material is mainly determined by the thermal conduction of phonons (energy quanta of lattice vibrations) and free electrons [2][11]. For polymers, regarded as thermal insulators, the thermal conductivity of polymers is dominated by the contribution of phonons, while in metals, the contribution from electrons is much greater than that of phonons [3][4][5,12]. According to Debye’s assumptions, the thermal conductivity (K) of polymers could be expressed as Equation:
Figure 1. Thermal conductivity of intrinsic thermally conductive polymers depends on various factors that need to be considered.
2. Molecular Chain Structure
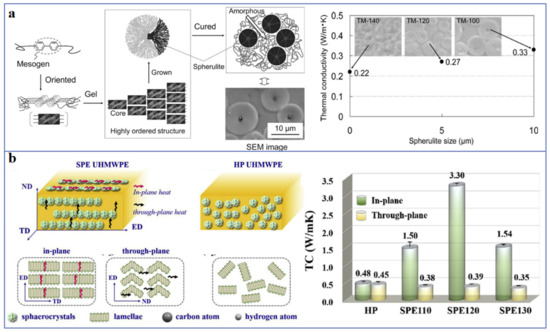
Polymers with rigid backbones have higher thermal conductivity, such as polyphenylene sulfide (PPS) [9][17], because the rigid backbone can inhibit the rotation of polymer chains and ameliorate the transmission of phonons. Conjugated π-bonded polymers also exhibit higher thermal conductivity, such as polyacetylene, polyaniline, polypyrrole, polythiophene, etc. This is due to the phonon heat conduction mechanism as well as the electron heat conduction mechanism. Zheng et al. [10][18] synthesized copolymers of 3-alkylthiophene and 3-alkoxythiophene with different p-π conjugation degree by oxidative polymerization. The results of laser measurement and molecular dynamics simulation show that the copolymers with high p-π conjugation degree have thermal conductivity as high as 0.374 Wm−1K−1. In addition, the introduction of small molecule monomers, liquid crystal structures or other regular structures in the synthesis of polymers can improve the microscopic order of the polymers, thus enhancing thermal conductivity [11][19]. As shown in Figure 2a, Song et al. [12][20] reported that the mesogenic epoxy resin maintains a high thermal conductivity of 0.33 Wm−1K−1, which is 1.7 times higher than that of amorphous epoxy resin, and the mesogenic epoxy resin with spherulite structure have a higher thermal conductivity of 1.16 Wm−1K−1. Recently, Ruan et al. [13][21] synthesized liquid crystalline polyimide (LC-PI) films using phthalimide groups as the mesomorphic units. The obtained LC-PI films with microscopically ordered molecular chains exhibit high thermal conductivity of 2.11 Wm−1K−1 (in-plane) and 0.32 Wm−1K−1 (through-plane).
3. Crystallinity and Crystal Morphology
Crystallization can increase the degree of structural order in polymer, so the thermal conductivity of crystalline polymers is generally higher than that of amorphous polymers, and the thermal conductivity of crystalline polymers increases with the increase of crystallinity [15][16][23,24]. Bai et al. [17][25] discussed the effect of crystallinity degree of poly-l-lactide (PLLA) on thermal conductivity. The results show that the thermal conductivity of PLLA increases from 0.16 Wm−1K−1 (amorphous PLLA) to 0.2 Wm−1K−1 when the crystallinity is 56%. Because the crystallinity of most polymers is less than 100%, there will inevitably be an interface between crystal and amorphous region, which will lead to phonon scattering and reduce the thermal conductivity. For instance, Huang et al. [14][22] reported that the ultrahigh molecular weight polyethylene (UHMWPE) has high thermal conductivity of 3.30 Wm−1K−1, which is due to the reduction of interface between crystals and amorphous regions through the formation of cylindrical crystals and highly oriented lamellae (Figure 2b). In addition, some studies show that the crystal morphology also has an important influence on the thermal conductivity of polymer. For example, the thermal conductivity of high-density polyethylene (HDPE) and UHMWPE with extended-chain crystals is higher than that of folded-chain lamella crystals [16][24].
4. Orientation of Molecular Chains
As mentioned above, the main factor causing the low thermal conductivity of the polymer is the random arrangement of the polymer molecular chains, because it is easier to conduct heat along the molecular chains rather than lateral direction. Therefore, the anisotropic thermal conductivity (especially the enhanced thermal conductivity along the stretching direction) can be achieved by aligning polymer molecular chains along a specific direction by mechanical stretching. So far, many experiments have studied the effect of orientation on the thermal conductivity of polymers, including crystalline and amorphous polymers [9][18][19][17,26,27].
For crystalline polymers, many studies have focused on polyethylene (PE). For example, Choy et al. [20][28] reported that when the drawing ratio is in the range of 1–25, the thermal conductivity of HDPE parallel to the stretching direction is much higher than that of HDPE perpendicular to the stretching direction in the temperature range of 120–320 K. To be specific, when the drawing ratio is 25, the thermal conductivity of HDPE parallel to the drawing direction is up to 8.5 W−1mK−1 (120 K) and 14 Wm−1K−1 (320 K). Choy et al. [21][29] further studied the effect of different drawing ratios (in the range of 1–350) on the thermal conductivity of PE, and the ultra-drawing PE fibers with the drawing ratio of 350 has a high thermal conductivity of 41.8 Wm−1K−1 in the drawing direction. This can be explained by the fact that when the drawing ratio increases, the crystal lamellae are broken into small crystal blocks and then rearranged to form microfibers; after further stretching, the microfibers are deformed into ordered long extended-chain crystals or even needle-like crystals. Shen et al. [22][30] also fabricated a series of ultra-stretched PE nanofibers, whose drawing ratio (60–800) is higher than that of Choy [21][29], and the highest thermal conductivity reaches 104 Wm−1K−1, which is almost close to the properties of PE single crystals.