Autism Spectrum Disorder (ASD) is a complex neurodevelopmental disorder characterized by persistent deficits in social communication and social interaction across multiple contexts and restricted, repetitive patterns of behavior, interests and activities. The maternal status of polyunsaturated fatty acids (PUFA) regulates microglial activity and neuroinflammatory pathways during a child’s brain development. In children with ASD, the metabolism of PUFA is thought to be deficient or abnormal, leading to increased production of proinflammatory cytokines, increased oxidative stress and an imbalance in the formation and action of neurotransmitters. In addition, nutritional deficits in omega-3 PUFA may affect gut microbiota and contribute to ASD by the gut–brain axis.
- ASD
- PUFA
- supplementation
- neuroinflammation
- gut microbiota
- gut–brain axis
1. Introduction
2. Gut–Brain Axis (GBA) and ASD
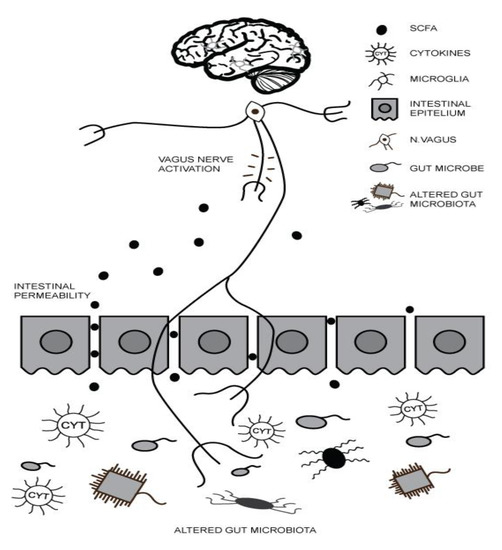
3. Maternal Inflammation during Pregnancy and ASD
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; New School Library: London, UK, 2013.
- Kohane, I.S.; McMurry, A.; Weber, G.; MacFadden, D.; Rappaport, L.; Kunkel, L.; Bickel, J.; Wattanasin, N.; Spence, S.; Murphy, S. The co-morbidity burden of children and young adults with autism spectrum disorders. PLoS ONE 2012, 7, e33224.
- Kraneveld, A.; Szklany, K.; de Theije, C.; Garssen, J. Gut-to-brain axis in autism spectrum disorders: Central role for the microbiome. Int. Rev. Neurobiol. 2016, 131, 263–287.
- Gaugler, T.; Klei, L.; Sanders, S.J.; Bodea, C.A.; Goldberg, A.P.; Lee, A.B.; Mahajan, M.; Manaa, D.; Pawitan, Y.; Reichert, J. Most genetic risk for autism resides with common variation. Nat. Genet. 2014, 46, 881–885.
- Carter, M.T.; Scherer, S.W. Autism spectrum disorder in the genetics clinic: A review. Clin. Genet. 2013, 83, 399–407.
- Castellani, M.; Conti, C.; Kempuraj, D.; Salini, V.; Vecchiet, J.; Tete, S.; Ciampoli, C.; Conti, F.; Cerulli, G.; Caraffa, A. Autism and immunity: Revisited study. Int. J. Immunopathol. Pharmacol. 2009, 22, 15–19.
- Frye, R.E.; Rose, S.; Slattery, J.; MacFabe, D.F. Gastrointestinal dysfunction in autism spectrum disorder: The role of the mitochondria and the enteric microbiome. Microb. Ecol. health Dis. 2015, 26, 27458.
- Vuong, H.E.; Hsiao, E.Y. Emerging roles for the gut microbiome in autism spectrum disorder. Biol. Psychiatry 2017, 81, 411–423.
- Li, Q.; Han, Y.; Dy, A.B.C.; Hagerman, R.J. The gut microbiota and autism spectrum disorders. Front. Cell. Neurosci. 2017, 11, 120.
- Noriega, D.B.; Savelkoul, H.F. Immune dysregulation in autism spectrum disorder. Eur. J. Pediatrics 2014, 173, 33–43.
- Ciéslińska, A.; Kostyra, E.; Savelkoul, H.F. Treating autism spectrum disorder with gluten-free and casein-free diet: The underlying microbiota-gut-brain axis mechanisms. HSOA J. Clin. Immunol. Immunother. 2017, 3, 11.
- Milošević, M.; Arsić, A.; Cvetković, Z.; Vučić, V. Memorable Food: Fighting Age-Related Neurodegeneration by Precision Nutrition. Front. Nutr. 2021, 8.
- Li, Q.; Zhou, J.-M. The microbiota–gut–brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience 2016, 324, 131–139.
- Iannone, L.F.; Preda, A.; Blottière, H.M.; Clarke, G.; Albani, D.; Belcastro, V.; Carotenuto, M.; Cattaneo, A.; Citraro, R.; Ferraris, C. Microbiota-gut brain axis involvement in neuropsychiatric disorders. Expert Rev. Neurother. 2019, 19, 1037–1050.
- MacFabe, D.F. Enteric short-chain fatty acids: Microbial messengers of metabolism, mitochondria, and mind: Implications in autism spectrum disorders. Microb. Ecol. health Dis. 2015, 26, 28177.
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286.
- Goines, P.E.; Ashwood, P. Cytokine dysregulation in autism spectrum disorders (ASD): Possible role of the environment. Neurotoxicol. Teratol. 2013, 36, 67–81.
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352.
- Madore, C.; Leyrolle, Q.; Lacabanne, C.; Benmamar-Badel, A.; Joffre, C.; Nadjar, A.; Layé, S. Neuroinflammation in autism: Plausible role of maternal inflammation, dietary omega 3, and microbiota. Neural Plast. 2016, 2016.
- Patterson, P.H. Maternal infection and immune involvement in autism. Trends Mol. Med. 2011, 17, 389–394.
- van der Burg, J.W.; Sen, S.; Chomitz, V.R.; Seidell, J.C.; Leviton, A.; Dammann, O. The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatric Res. 2016, 79, 3–12.
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2005, 57, 67–81.
