Myocardial infarction (MI) occurs when the coronary blood supply is interrupted. As a consequence, cardiomyocytes are irreversibly damaged and lost. Unfortunately, current therapies for MI are unable to prevent progression towards heart failure. As the renewal rate of cardiomyocytes is minimal, the optimal treatment should achieve effective cardiac regeneration, possibly with stem cells transplantation. In that context, our research group identified the cardiac atrial appendage stem cells (CASCs) as a new cellular therapy. However, CASCs are transplanted into a hostile environment, with elevated levels of advanced glycation end products (AGEs), which may affect their regenerative potential. In this study, we hypothesize that pyridoxamine (PM), a vitamin B6 derivative, could further enhance the regenerative capacities of CASCs transplanted after MI by reducing AGEs’ formation. Methods and Results: MI was induced in rats by ligation of the left anterior descending artery. Animals were assigned to either no therapy (MI), CASCs transplantation (MI + CASCs), or CASCs transplantation supplemented with PM treatment (MI + CASCs + PM). Four weeks post-surgery, global cardiac function and infarct size were improved upon CASCs transplantation. Interstitial collagen deposition, evaluated on cryosections, was decreased in the MI animals transplanted with CASCs. Contractile properties of resident left ventricular cardiomyocytes were assessed by unloaded cell shortening. CASCs transplantation prevented cardiomyocyte shortening deterioration. Even if PM significantly reduced cardiac levels of AGEs, cardiac outcome was not further improved. Conclusion: Limiting AGEs’ formation with PM during an ischemic injury in vivo did not further enhance the improved cardiac phenotype obtained with CASCs transplantation. Whether AGEs play an important deleterious role in the setting of stem cell therapy after MI warrants further examination.
- stem cells
- CASCs
- advanced glycation end products
- glycated proteins
- myocardial infarction
- transplantation
- remodeling
- cardiomyocytes
- aldehyde dehydrogenase
1. Introduction
2. Analysis on Results
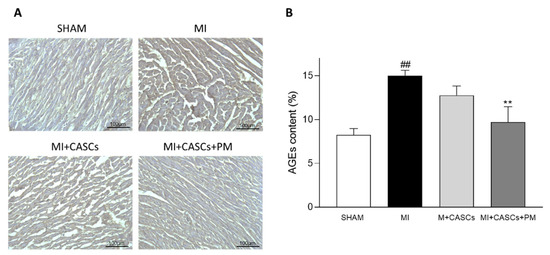
2.1. AGEs’ Levels Are Reduced with PM Treatment
2.2. CASCs Transplantation Prevents Loss of LV Function after MI
| Parameters | 4 Weeks Post-Operative | |||
|---|---|---|---|---|
| SHAM | MI | MI + CASCs | MI + CASCs + PM | |
| Parameters | 4 Weeks Post-Operative | ||||||
|---|---|---|---|---|---|---|---|
| SHAM | MI | MI + CASCs | MI + CASCs + PM | ||||
| EF (%) | 80 ± 4 | 59 ± 4 ## | 79 ± 3 *** | 72 ± 3 | |||
| HR (bpm) | 333 ± 14 | 318 ± 12 | 332 ± 12 | 327 ± 13 | |||
| SV (µL) | |||||||
| Max LV pressure (mmHg) | 99 ± 3 | 90 ± 2 | 96 ± 3 | 103 ± 4 * | |||
| 170 ± 16 | 162 ± 17 | 172 ± 17 | 192 ± 17 | ||||
| CO (mL/min) | 57 ± 4 | 51 ± 5 | 59 ± 6 | 65 ± 6 | |||
| EDV (µL) | 215 ± 26 | ||||||
| dP/dtmax (mmHg/s) | 6773 ± 529 | 6038 ± 242 | 6923 ± 340 | 6551 ± 257 | |||
| dP/dtmin (mmHg/s) | −7269 ± 683 | −6550 ± 705 | −6816 ± 354 | −6917 ± 273 | |||
| Tau (s) | 0.0130 ± 0.001 | 0.0499 ± 0.017 | 0.0148 ± 0.002 | 0.0117 ± 0.001 ** | 298 ± 51 | 217 ± 17 | 267 ± 22 |
| ESV (µL) | 45 ± 13 | 136 ± 36 | 44 ± 5 * | 77 ± 11 | |||
| AWT (mm) | 1.74 ± 0.13 | 1.55 ± 0.16 | 1.65 ± 0.12 | 1.80 ± 0.16 | |||
| PWT (mm) | 1.54 ± 0.16 | 1.63 ± 0.15 | 1.57 ± 0.09 | 1.62 ± 0.17 | |||
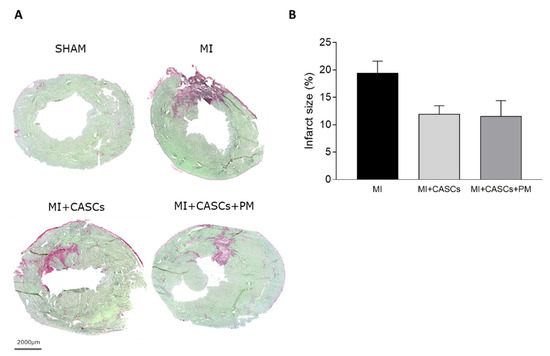
2.3. CASCs Transplantation Tended to Reduce Infarct Size
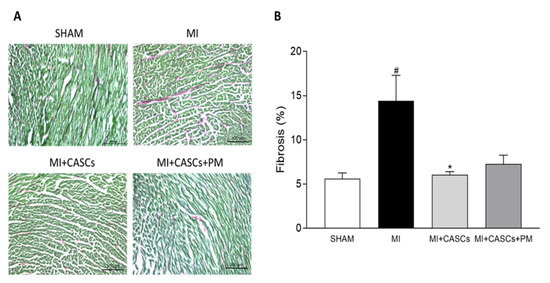
2.4. CASCs Transplantation Prevents the Increased Interstitial Collagen Deposition Seen with MI
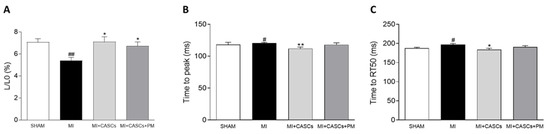
2.5. CASCs Transplantation Prevents Resident Cardiomyocyte Functional Remodeling
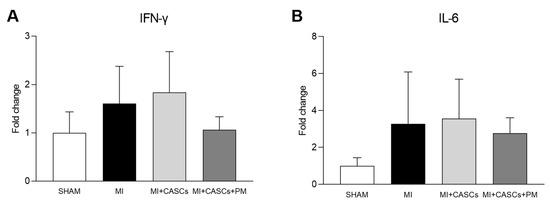
2.6. PM Treatment Tended to Reduced Tissue Pro-Inflammatory Cytokine Levels
3. Current Insights
3.1. Combining CASCs and PM to Enhance Cardiac Repair
3.2. CASCs Alone Are an Effective Therapy for MI
References
- Koninckx, R.; Daniels, A.; Windmolders, S.; Mees, U.; Macianskiene, R.; Mubagwa, K.; Steels, P.; Jamaer, L.; Dubois, J.; Robic, B.; et al. The cardiac atrial appendage stem cell: A new and promising candidate for myocardial repair. Cardiovasc. Res. 2013, 97, 413–423.
- Fanton, Y.; Robic, B.; Rummens, J.L.; Daniels, A.; Windmolders, S.; Willems, L.; Jamaer, L.; Dubois, J.; Bijnens, E.; Heuts, N.; et al. Cardiac atrial appendage stem cells engraft and differentiate into cardiomyocytes in vivo: A new tool for cardiac repair after MI. Int. J. Cardiol. 2015, 201, 10–19.
- Kralev, S.; Zimmerer, E.; Brueckmann, M.; Lang, S.; Kalsch, T.; Rippert, A.; Lin, J.; Borggrefe, M.; Hammes, H.P.; Suselbeck, T. Elevation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in patients presenting with acute myocardial infarction. Clin. Chem. Lab. Med. 2009, 47, 446–451.
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146.
- Hartog, J.W.; Voors, A.A.; Bakker, S.J.; Smit, A.J.; van Veldhuisen, D.J. Advanced glycation end-products (AGEs) and heart failure: Pathophysiology and clinical implications. Eur. J. Heart Fail. 2007, 9, 1146–1155.
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. Stopping the primal RAGE reaction in myocardial infarction: Capturing adaptive responses to heal the heart? Circulation 2008, 117, 3165–3167.
- Liu, Y.; Qu, Y.; Wang, R.; Ma, Y.; Xia, C.; Gao, C.; Liu, J.; Lian, K.; Xu, A.; Lu, X.; et al. The alternative crosstalk between RAGE and nitrative thioredoxin inactivation during diabetic myocardial ischemia-reperfusion injury. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E841–E852.
- Fishman, S.L.; Sonmez, H.; Basman, C.; Singh, V.; Poretsky, L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol. Med. 2018, 24, 59.
- Yang, J.; Zhang, F.; Shi, H.; Gao, Y.; Dong, Z.; Ma, L.; Sun, X.; Li, X.; Chang, S.; Wang, Z.; et al. Neutrophil-derived advanced glycation end products-Nepsilon-(carboxymethyl) lysine promotes RIP3-mediated myocardial necroptosis via RAGE and exacerbates myocardial ischemia/reperfusion injury. FASEB J. 2019, 33, 14410–14422.
- Son, M.; Kang, W.C.; Oh, S.; Bayarsaikhan, D.; Ahn, H.; Lee, J.; Park, H.; Lee, S.; Choi, J.; Lee, H.S.; et al. Advanced glycation end-product (AGE)-albumin from activated macrophage is critical in human mesenchymal stem cells survival and post-ischemic reperfusion injury. Sci. Rep. 2017, 7, 11593.
- Evens, L.; Belien, H.; Deluyker, D.; Bronckaers, A.; Gervois, P.; Hendrikx, M.; Bito, V. The Impact of Advanced Glycation End-Products (AGEs) on Proliferation and Apoptosis of Primary Stem Cells: A Systematic Review. Stem Cells Int. 2020, 2020, 8886612.
- Evens, L.; Heeren, E.; Rummens, J.L.; Bronckaers, A.; Hendrikx, M.; Deluyker, D.; Bito, V. Advanced Glycation End Products Impair Cardiac Atrial Appendage Stem Cells Properties. J. Clin. Med. 2021, 10, 2964.
- Ramis, R.; Ortega-Castro, J.; Caballero, C.; Casasnovas, R.; Cerrillo, A.; Vilanova, B.; Adrover, M.; Frau, J. How Does Pyridoxamine Inhibit the Formation of Advanced Glycation End Products? The Role of Its Primary Antioxidant Activity. Antioxidants 2019, 8, 344.
- Voziyan, P.A.; Hudson, B.G. Pyridoxamine as a multifunctional pharmaceutical: Targeting pathogenic glycation and oxidative damage. Cell. Mol. Life Sci. 2005, 62, 1671–1681.
- Paul, L.; Ueland, P.M.; Selhub, J. Mechanistic perspective on the relationship between pyridoxal 5’-phosphate and inflammation. Nutr. Rev. 2013, 71, 239–244.
- Lemcke, H.; Voronina, N.; Steinhoff, G.; David, R. Recent Progress in Stem Cell Modification for Cardiac Regeneration. Stem Cells Int. 2018, 2018, 1909346.
- Qiu, H.; Li, W.P.; Shen, X.H.; Guo, X.Y.; Hua, B.; Li, H.W. Dynamic fluctuations of advanced glycation end products and its C-terminal truncated receptor level in patients with acute ST-segment elevation myocardial infarction and undergoing diabetes or not: A retrospective study. Medicine 2018, 97, e11278.
- Greven, W.L.; Smit, J.M.; Rommes, J.H.; Spronk, P.E. Accumulation of advanced glycation end (AGEs) products in intensive care patients: An observational, prospective study. BMC Clin. Pathol. 2010, 10, 4.
- Celec, P.; Hodosy, J.; Jani, P.; Janega, P.; Kudela, M.; Kalousova, M.; Holzerova, J.; Parrak, V.; Halcak, L.; Zima, T.; et al. Advanced glycation end products in myocardial reperfusion injury. Heart Vessel. 2012, 27, 208–215.
- Dwyer, J.P.; Greco, B.A.; Umanath, K.; Packham, D.; Fox, J.W.; Peterson, R.; Broome, B.R.; Greene, L.E.; Sika, M.; Lewis, J.B. Pyridoxamine dihydrochloride in diabetic nephropathy (PIONEER-CSG-17): Lessons learned from a pilot study. Nephron 2015, 129, 22–28.
- Voziyan, P.A.; Hudson, B.G. Pyridoxamine: The many virtues of a maillard reaction inhibitor. Ann. N. Y. Acad. Sci. 2005, 1043, 807–816.
- Williams, M.E. Clinical studies of advanced glycation end product inhibitors and diabetic kidney disease. Curr. Diabetes Rep. 2004, 4, 441–446.
- Deluyker, D.; Ferferieva, V.; Driesen, R.B.; Verboven, M.; Lambrichts, I.; Bito, V. Pyridoxamine improves survival and limits cardiac dysfunction after MI. Sci. Rep. 2017, 7, 16010.
- Borg, D.J.; Forbes, J.M. Targeting advanced glycation with pharmaceutical agents: Where are we now? Glycoconj. J. 2016, 33, 653–670.
- Vagnozzi, R.J.; Maillet, M.; Sargent, M.A.; Khalil, H.; Johansen, A.K.Z.; Schwanekamp, J.A.; York, A.J.; Huang, V.; Nahrendorf, M.; Sadayappan, S.; et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 2020, 577, 405–409.
- Oh, S.; Son, M.; Choi, J.; Lee, S.; Byun, K. sRAGE prolonged stem cell survival and suppressed RAGE-related inflammatory cell and T lymphocyte accumulations in an Alzheimer’s disease model. Biochem. Biophys. Res. Commun. 2018, 495, 807–813.
- Park, M.J.; Lee, S.H.; Moon, S.J.; Lee, J.A.; Lee, E.J.; Kim, E.K.; Park, J.S.; Lee, J.; Min, J.K.; Kim, S.J.; et al. Overexpression of soluble RAGE in mesenchymal stem cells enhances their immunoregulatory potential for cellular therapy in autoimmune arthritis. Sci. Rep. 2016, 6, 35933.
- Lee, J.; Bayarsaikhan, D.; Arivazhagan, R.; Park, H.; Lim, B.; Gwak, P.; Jeong, G.B.; Lee, J.; Byun, K.; Lee, B. CRISPR/Cas9 Edited sRAGE-MSCs Protect Neuronal Death in Parkinsons Disease Model. Int. J. Stem Cells 2019, 12, 114–124.
- Lang, C.I.; Wolfien, M.; Langenbach, A.; Muller, P.; Wolkenhauer, O.; Yavari, A.; Ince, H.; Steinhoff, G.; Krause, B.J.; David, R.; et al. Cardiac Cell Therapies for the Treatment of Acute Myocardial Infarction: A Meta-Analysis from Mouse Studies. Cell. Physiol. Biochem. 2017, 42, 254–268.
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-beta signaling in fibrosis. Growth Factors 2011, 29, 196–202.
- Frangogiannis, N.G. The role of transforming growth factor (TGF)-beta in the infarcted myocardium. J. Thorac. Dis. 2017, 9, S52–S63.
- Voloshenyuk, T.G.; Landesman, E.S.; Khoutorova, E.; Hart, A.D.; Gardner, J.D. Induction of cardiac fibroblast lysyl oxidase by TGF-beta1 requires PI3K/Akt, Smad3, and MAPK signaling. Cytokine 2011, 55, 90–97.
- Jin, L.; Zhang, J.; Deng, Z.; Liu, J.; Han, W.; Chen, G.; Si, Y.; Ye, P. Mesenchymal stem cells ameliorate myocardial fibrosis in diabetic cardiomyopathy via the secretion of prostaglandin E2. Stem Cell Res. Ther. 2020, 11, 122.
- Richardson, W.J.; Clarke, S.A.; Quinn, T.A.; Holmes, J.W. Physiological Implications of Myocardial Scar Structure. Compr. Physiol. 2015, 5, 1877–1909.
- Kanda, P.; Davis, D.R. Cellular mechanisms underlying cardiac engraftment of stem cells. Expert Opin. Biol. Ther. 2017, 17, 1127–1143.
- Baraniak, P.R.; McDevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010, 5, 121–143.
- Wu, S.Z.; Li, Y.L.; Huang, W.; Cai, W.F.; Liang, J.; Paul, C.; Jiang, L.; Wu, Z.C.; Xu, M.; Zhu, P.; et al. Paracrine effect of CXCR4-overexpressing mesenchymal stem cells on ischemic heart injury. Cell Biochem. Funct. 2017, 35, 113–123.
- Dulak, J.; Szade, K.; Szade, A.; Nowak, W.; Jozkowicz, A. Adult stem cells: Hopes and hypes of regenerative medicine. Acta Biochim. Pol. 2015, 62, 329–337.
