Dicarboxymethyl cellulose (DCMC) is a cellulose derivative prepared via heterogeneous catalysed etherification. The polymer is negatively charged at wine pH due to the presence of malonic groups, which makes it suitable for removing positively charged compounds such as dyes. DCMC has similar structure and properties to carboxymethyl cellulose (CMC), a compound commonly used for wine tartrate stabilization. CMC, having an acidic group with a pKa near 4.5, becomes mostly uncharged at a pH below 3.5, which is common in white wines, failing to promote ionic exchange and remove positively charged proteins. Nevertheless, the malonic carboxylate groups are more acidic than the one present in CMC and, consequently, more prone to be deprotonated at wine pH due to its first pKa (approx. 3).
One of the most prevalent causes of white wine haze occurs from the aggregation and denaturation of grape pathogenesis-related proteins, namely thaumatin-like (TLP) and chitinase proteins. The conditions associated with protein haze are the exposure of wines to high temperatures and long-term storage. To avoid this phenomenon, proteins are frequently removed through the negatively charged clay bentonite. Dicarboxymethyl cellulose (DCMC) can be a substitute for this non-renewable material.
- bentonite
- dicarboxymethyl cellulose
- wine protein haze
- cellulose derivative
- sustainability
1. Overview
Wine clarity is a critical aspect in the commercialization of white wines. The formation of wine haze can be attributed to the aggregation and precipitation of heat-unstable wine proteins. Bentonite fining is the commonly used method in winemaking for protein removal, but it is responsible for loss of wine volume and quality. Dicarboxymethyl cellulose (DCMC) was developed as a potential alternative to bentonite. Water-insoluble DCMC was prepared via catalysed heterogeneous etherification using sodium chloromalonate and potassium iodide. White wine fining trials were benchmarked with different dosages of DCMC against bentonite. A high-performance liquid chromatography method was optimized for protein quantification. The samples underwent heat stability tests to evaluate wine turbidity before and after fining. Results show that DCMC successfully reduced the wine protein content and turbidity. DCMC produced heat-stable wines with dosages higher than 0.25 g/L. The innovative application of DCMC in the wine sector shows potential due to its ability to stabilize white wines while overcoming problems associated with bentonite, such as lees production and loss of wine, contributing to a more sustainable process.
2. Dicarboxymethyl cellulose
3. Wine Fining Trials
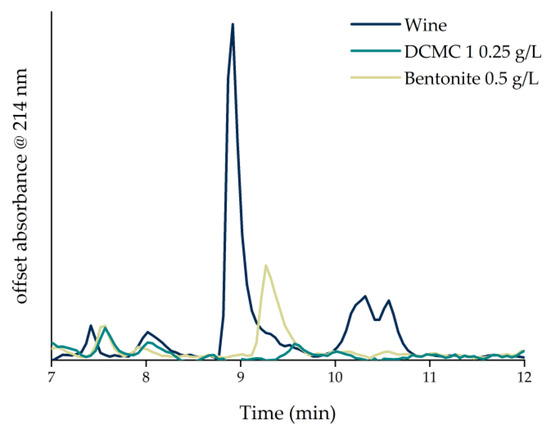
The effect of heating on the wine samples was investigated by performing a heat stability test (HST). Differences in wine turbidity (before and after the heat treatment) have been shown to correlate directly with wine protein instability [9]. In practical terms, a wine is considered unstable when the difference in absorbance between heated and unheated controls is higher than 0.02 absorbance units [10]. Following the HST, the control wine was considered unstable since the Δabs at 540 nm was of 0.23 ± 0.0011. The wine produced a significant amount of haze and was, therefore, considered heat-unstable. The adsorption experiments were carried out by adding different amounts of dicarboxymethyl cellulose sodium salt or bentonite to the sample wine. The experiments were conducted in 15 mL falcons placed horizontally in an orbital shaker, since DCMC sediments easily and this display allows better contact between wine and DCMC. Figure 1 shows the result of the HST applied to the sample wine after contact with the stabilizing agents. The heat stability test provides information on the haze potential of the wine based on the turbidity data. A correlation between adsorbent concentration and wine haze is evident. With increasing dosages, the turbidity of the resulting wine is reduced. Based on the heat test, wines treated with all concentrations of DCMC 02 remained unstable, whereas wines treated with more than 0.25 g/L of DCMC 01 were stabilized. Bentonite fining required more than 0.5 g/L to stabilize the wine. The difference in adsorption capacity for the two DCMC samples can be explained by the number of carboxylate groups present on the polymer. Increasing the molar equivalents of the etherification agent increases the degree of substitution. An increase in the degree of substitution (DS of 0.03 and 0.01 for DCMC 1 and 2, respectively) allowed for a higher adsorption of positively charged wine proteins due to the increased availability of binding sites. Therefore, DCMC promoted wine stabilization as verified by the heat stability test. Contrarily to other methods described in the literature, DCMC can be used in very low doses and without heating the samples [2][7].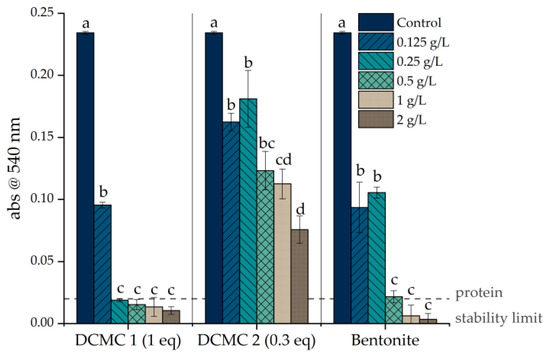
4. Conclusions
References
- Romanini, E.; McRae, J.M.; Bilogrevic, E.; Colangelo, D.; Gabrielli, M.; Lambri, M. Use of grape seeds to reduce haze formation in white wines. Food Chem. 2021, 341, 128250.
- Mierczynska-Vasilev, A.; Mierczynski, P.; Maniukiewicz, W.; Visalakshan, R.M.; Vasilev, K.; Smith, P.A. Magnetic separation technology: Functional group efficiency in the removal of haze-forming proteins from wines. Food Chem. 2019, 275, 154–160.
- Vincenzi, S.; Panighel, A.; Gazzola, D.; Flamini, R.; Curioni, A. Study of Combined Effect of Proteins and Bentonite Fining on the Wine Aroma Loss. J. Agric. Food Chem. 2015, 63, 2314–2320.
- Sommer, S.; Tondini, F. Sustainable Replacement Strategies for Bentonite in Wine Using Alternative Protein Fining Agents. Sustainability 2021, 13, 1860.
- Colangelo, D.; Torchio, F.; De Faveri, D.M.; Lambri, M. The use of chitosan as alternative to bentonite for wine fining: Effects on heat-stability, proteins, organic acids, colour, and volatile compounds in an aromatic white wine. Food Chem. 2018, 264, 301–309.
- Sui, Y.; McRae, J.M.; Wollan, D.; Muhlack, R.A.; Godden, P.; Wilkinson, K.L. Use of ultrafiltration and proteolytic enzymes as alternative approaches for protein stabilisation of white wine. Aust. J. Grape Wine Res. 2021, 27, 234–245.
- Marangon, M.; Vincenzi, S.; Curioni, A. Wine Fining with Plant Proteins. Molecules 2019, 24, 2186.
- Pachova, V.; Ferrando, M.; Güell, C.; López, F. Protein adsorption onto metal oxide materials in white wine model systems. J. Food Sci. 2002, 67, 2118–2121.
- Pocock, K.F.; Waters, E.J. Protein haze in bottled white wines: How well do stability tests and bentonite fining trials predict haze formation during storage and transport? Aust. J. Grape Wine R. 2006, 12, 212–220.
- Marangon, M.; Van Sluyter, S.C.; Haynes, P.A.; Waters, E.J. Grape and Wine Proteins: Their Fractionation by Hydrophobic Interaction Chromatography and Identification by Chromatographic and Proteomic Analysis. J. Agric. Food. Chem. 2009, 57, 4415–4425.
