You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by MARIA Rosa Ciriolo.
Glutathione (GSH) is the main non-enzymatic antioxidant playing an important role in detoxification and signal transduction by modulation of protein thiols redox status and direct scavenging of radicals. The latter function is not only performed against reactive oxygen species (ROS) but GSH also has a fundamental role in buffering nitric oxide (NO), a physiologically-produced molecule having multifaceted functions. The efficient rate of GSH synthesis and high levels of GSH-dependent enzymes are characteristic features of healthy skeletal muscle where, besides the canonical functions, it is also involved in muscle contraction regulation.
- exercise
- oxidative stress
- nitrosative stress
- inflammation
1. Introduction
γ-l-glutamyl-l-cysteinyl-glycine (glutathione, GSH) is the most versatile non-enzymatic antioxidant involved in cell signaling, detoxification and oxy-radical scavenging activity. Its structure, cellular concentration and the systems deputed to its synthesis, degradation and regeneration concur to these different functions. For instance: (i) GSH synthesis is a two-step process mediated by cytosolic enzymes (a faster process with respect to a gene-mediated one); (ii) the peculiar iso-peptide bond of the glutamate makes GSH a protease-resistant peptide; (iii) the NADPH-reductase systems guarantee the maintaining of a high ratio between reduced (GSH) and oxidized (GSSG, GSSR) forms under physiological conditions. Alteration of such ratio during oxidative burst can modulate redox signaling pathways related to various cellular processes including proliferation, growth and apoptosis [1].
Two ATP-dependent cytosolic enzymes are responsible for the de novo synthesis of GSH: glutamate-cysteine ligase (GCL) also named γ-glutamylcysteine synthase (GCS) and glutathione synthase (GS). The first is the rate-limiting enzyme formed by a modulatory or light subunit (GCLM) and a catalytic or heavy subunit (GCLC). Using ATP hydrolysis, GCLC is able to shape an iso-peptide bond between the γ-carboxyl of glutamate and the amino group of cysteine. GS catalyzes the addition of glycine to γ-glutamylcysteine created by GCLC to form GSH [1]. GCLC is transcriptionally regulated by Nuclear factor (erythroid-derived 2)-like 2 (NFE2L2), a transcription factor that regulates a wide array of antioxidant responsive element-driven genes in various cell types [2]. GSH degradation occurs in the extracellular space by the enzyme γ-glutamyl-transpeptidase (γ-GT), which cleaves GSH into cysteinyl-glycine (Cys-Gly) and glutamate. Thus, systemic GSH homeostasis relies on its intracellular synthesis, export and degradation outside the cell in a cycle with a half-life of 2–3 h [3]. Intracellularly, GSH is present in a dynamic equilibrium with its oxidized forms, mainly GSSG, which is the product of a disulfide bridge between two molecules of GSH directly produced upon oxidative burst or by the activity of the antioxidant enzyme glutathione peroxidase (GPx). Subsequently, GSSG is converter to GSH through the glutathione reductase (GR). GSSR represents a less concentrated form deriving from mixed disulfide formation with protein thiols (protein S-glutathionylation); however, this oxidized form is pivotal in redox signaling pathways representing a reversible post-translational modification of proteins [4]. The value of GSH/GSSG ratio is universally accepted as an index of cell redox status because of the scavenging effect of GSH against several reactive oxygen species (e.g., hydroxyl radical: OH, superoxide anion: O2−, hydrogen peroxide: H2O2, lipid peroxyl radical: HOO), either directly or through the activity of antioxidant enzymes, mainly the glutathione peroxidases. GSH homeostasis is fundamental for cell metabolism and function as stated by the association of altered GSH levels with numerous conditions and pathologies such as aging, neurodegeneration, cancer, inflammation and muscle degeneration (Figure 1) [5,6,7,8][5][6][7][8].
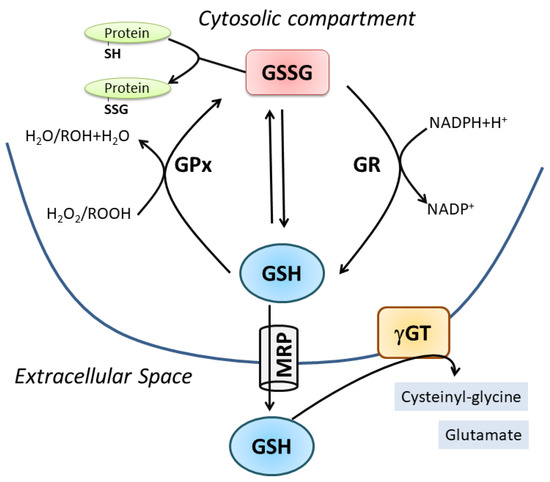
Figure 1. Cellular GSH homeostasis. For details see the text.
GSH plays an important role also in the field of nitric oxide (NO) physiology and pathology [9,10,11][9][10][11]. NO is a gaseous free radical synthesized by NO synthases (NOSs) starting from L-arginine, NADPH and oxygen. NO is a highly reactive molecule that despite being potentially toxic is implicated in a wide range of physiological processes, such as neurotransmission, vascular tone regulation, musculature contraction/relaxation and platelet aggregation [12,13,14][12][13][14]. Furthermore, it is an integral part of the inflammatory response to pathogens and cancer cells [15,16][15][16]. This versatility of NO mainly derives from its complex chemistry. Depending on its distribution and reactants, NO can exist in different redox forms with distinct and highly reactive properties, such as nitroxyl (HNO), oxides (NO2, N2O4 and N2O3), peroxynitrite (ONOO·−) and S-nitrosothiols (RSNO) [17].
GSH and NO can easily interact at different levels; a number of still debated mechanisms commit NO to react with GSH with the formation of S-nitrosoglutathione (GSNO), which is considered an endogenous NO reserve that contributes to the intracellular regulation of nitrosative stress [18]. In fact, we and other researchers demonstrated that neuronal NOS (nNOS) overexpression or treatments with NO donors result in intracellular GSH increase, a process fundamental to counteract neuronal death [11,19,20][11][19][20]. Thiol of “reactive cysteine” on proteins (P-SH) is another important target of reactive oxygen/nitrogen species (ROS/RNS) signaling pathway because it can be oxidized at a different extent up to the sulfonic form (P-SO3H), an irreversible oxidation product. On the contrary, the sulfenic form (P-SOH) represents the most frequent and reversible modification under physiological ROS/RNS flux. It can react with NO to produce the S-nitrosylation derivative (P-SNO), which seems to be implicated in the majority of long-range NO-cellular redox processes. The P-SNO can be reconverted to P-SH by thiol-dependent systems, among which GSH plays a prominent role through the translocation reaction of NO that generates GSNO [18,21][18][21] (Figure 2). Based on this evidence, it is conceivable to link the majority of NO effects on the availability and redox status of GSH.
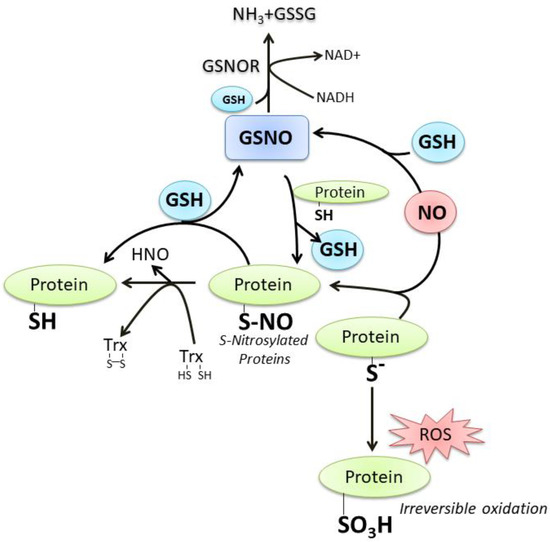
Figure 2. The crosstalk between GSH and NO. For details see the text.
2. Oxidative/Nitrosative-Mediated Signaling and Stress as the Conditions Where NO Mainly Intersects GSH
Oxidative/nitrosative stress is properly “an imbalance between oxidants and antioxidants in favor of the oxidant species, potentially leading to damage” [26,27][22][23]. ROS are by-products of the sequential one-electron reduction of molecular oxygen, which includes radical and non-radical species. RNS are a group of reactive compounds that include NO, which is physiologically produced by NOSs, a NADPH-dependent family of enzymes present in most cells and tissues [28][24]. To date, three main isoenzymes belonging to this family have been cloned and purified: nNOS, endothelial NOS (eNOS) and inducible NOS (iNOS) [29][25]. The first two are constitutively expressed and calcium (Ca2+)-dependent enzymes. nNOS is mainly expressed in cells of neuronal origin where NO functions as a neuromodulator and neurotransmitter in the central and peripheral nervous system. Notably, the nNOS isoform is also present at the skeletal and cardiac muscle level, namely the nNOSμ [30,31][26][27]. It is characterized by a 34-amino acid insert that arises from the alternative splicing of nNOS pre-mRNA between exons 16 and 17. The catalytic activity is similar to that of nNOS and its expression coincides with the formation of differentiated myotube in cultured cells. eNOS is localized in the endothelium, in cardiomyocytes and adipocytes. It principally participates in the regulation of blood pressure and vascular tone. Instead, iNOS is a Ca2+-independent and cytokine-inducible enzyme, expressed in macrophages during inflammation and tissue injury [32][28]. In recent years, the importance of the fourth isoform of NOS has also emerged: the mitochondrial NOS (mtNOS) [33][29]. It is localized in mitochondria of several tissues, including skeletal muscle, where it regulates O2 consumption [34][30].
NO is a hydrophobic gas molecule with a high diffusion coefficient but a short lifetime mainly due to its rapid reaction with oxygen and multiple cellular components. Therefore, as in the case of ROS, low and regulated production of NO is associated with paracrine signaling processes whereas its overproduction can cause damage to biological macromolecules and determine many pathological states [18,23,35][18][31][32]. In this regard, the product of NO reaction with O2− leads to the formation of ONOO·−, which is a strong oxidant that reacts with lipids, DNA, and proteins via direct oxidative reactions or via indirect, radical-mediated mechanisms [36][33].
3. GSH/NO Crosstalk in Skeletal Muscle Physiology
Skeletal muscle represents the most important district of the body where fluctuations of ROS/RNS could be observed in relation to physiological function. Muscle cell homeostasis is strictly dependent on regulated NO production by nNOS localized at the acetylcholine receptor [47][34]. Consistently, NO is involved in myotube differentiation and synaptic signaling from nerve to myotube. An increase in ROS production also occurs during differentiation due to the dramatic increase of NADPH oxidases activity [48][35]. On the other side, myoblasts derived from GPx1 knockout (Gpx1-/-) mice scarcely differentiate leading to only a few myotubes with respect to wild-type cells [49][36].
Physical activity of the skeletal muscle is directly correlated with O2 consumption, therefore, more intense is the exercise greater is the production of ROS/RNS. Moreover, such changes could be very fast due to conversion from rest to intense muscle contraction. This could explain the various and efficient antioxidant systems at the muscle level including various isoenzymes of Superoxide dismutase (SOD), GPx and catalase, high activity of GSH-dependent enzymes and a strong ability to synthesize new GSH [24,50][37][38]. Additionally, GSH levels in skeletal muscle are related to the metabolic profile of the tissue; in healthy human skeletal muscle fibers, the level of GSH is higher in aerobic type I fibers than in anaerobic type II fibers [51][39]. There are several potential sources of ROS/RNS in skeletal muscle but also other organs can participate in their production during exercise. However, because of the invasive nature of analyses used to determine antioxidants variations, the majority of the studies during exercise in humans evaluated blood markers, whereas data at muscle level were mainly from animals exposed to exercise training. As generally accepted, mitochondria represent the major source of ROS, but during exercise at skeletal muscle this is still debated since mitochondria produce more ROS under state 4 (basal) with respect to state 3 (maximum rate of ADP-stimulated respiration), the latter being the condition under aerobic muscle contraction [40]. Additional sources, as above mentioned, include NADPH-oxidase enzymes located at the different membrane structures (sarcoplasmic reticulum, plasma membrane, transverse tubule), phospholipase A2 and xanthine oxidase [38]. Changes in expression/activity of antioxidant enzymes, such during exercise at skeletal muscle since mitochondria produce more ROS under state 4 (basal) with respect to state 3 (maximum rate of ADP-stimulated respiration), the latter being the condition under aerobic muscle contraction [52]. Additional sources, as above mentioned, include NADPH-oxidase enzymes located at the different membrane structures (sarcoplasmic reticulum, plasma membrane, transverse tubule), phospholipase A2 and xanthine oxidase [50]. Changes in expression/activity of antioxidant enzymes, such as SOD and GPx, were deeply investigated both in humans and animals during training; their increases are muscle-specific with higher effect in oxidative fibers also at mitochondrial level [24,51]. Controversial results were reported for catalase activity because the majority of the studies in humans determined the level of Has SOD and GPx, were deeply investigated both in humans and animals during training; their increases are muscle-specific with higher effect in oxidative fibers also at mitochondrial level [37][39]. Controversial results were reported for catalase activity because the majority of the studies in humans determined the level of H2O2, a very unstable substrate. However, catalase was found to be increased in the gastrocnemius of mice upon prolonged exercise [53]; instead, GSH was always affected in all muscle types and increased levels of the tripeptide characterized trained muscle [54]. Recently, a metabolome approach was used in order to characterize the effects of exercise on human skeletal muscle. The results showed that vigorous exercise leads to GSSG and RSSG production as a consequence of increased oxidative stress [55]., a very unstable substrate. However, catalase was found to be increased in the gastrocnemius of mice upon prolonged exercise [41]; instead, GSH was always affected in all muscle types and increased levels of the tripeptide characterized trained muscle [42]. Recently, a metabolome approach was used in order to characterize the effects of exercise on human skeletal muscle. The results showed that vigorous exercise leads to GSSG and RSSG production as a consequence of increased oxidative stress [43].
Behind the detrimental role of excessive ROS, recent evidence has documented that low ROS levels produced during exercise have positive effects by influencing cellular adaptation [56][44]. Protein S-glutathionylation represents a reversible and ubiquitous process important for avoiding excessive oxidation of thiol-derivatives of protein cysteine under oxidative stress. The glutathionylation process is mediated by Trx or other “redoxins” and is responsible for protein structure/function changes, therefore, resulting in the modulation of several cellular metabolic processes [4]. Heart and skeletal muscle contraction and metabolism are regulated by such process through modulation of several ions pumps and contractile proteins [57,58][45][46]. In fact, mammalian fast-twitch (i.e., type II) muscle fibers exposed to GSH and reactant to induce S-glutathionylation showed an increase in their sensitivity to Ca2+, which was abrogated by the reducing agent dithiothreitol (DTT), indicating not only modification of protein thiols, but also that glutathionylation of skeletal muscle proteins can modulate Ca2+ sensitivity [59][47]. Moreover, the authors reported that the protein responsible for such an effect was the fast troponin I, which can be S-glutathionylated on Cys134. The most intriguing evidence concerning this protein is that the same Cys residue can be also S-nitrosylated with a concomitant decrease in Ca2+ sensitivity. This study provides unambiguous evidence on the cross-talk between GSH and NO in muscle functionality: NO counterbalances the ability of oxidant-induced S-glutathionylation to increase Ca2+ sensitivity, a condition that if persistent would adversely affect skeletal mass performance [60][48]. Skeletal muscle mitochondria subjected to in vitro treatment with compounds inducing S-glutathionylation (diamide or disulfiram) produced less ROS as a by-product of aerobic oxidation of either carbohydrates or fatty acid derivatives. This inhibition was due to impaired pyruvate uptake by deactivation of mitochondrial pyruvate carrier and S-glutathionylation of Complex I [61][49]. This regulatory process is fundamental in avoiding increased ROS burst that can result in irreversible protein oxidation and mitochondrial damage. The GSH mediated reversible oxidation of specific proteins directly related to ROS production could have a twofold benefit: on one hand, it lowers the harmful effects of high ROS flux and on the other hand, it allows the restoration of protein functions once the intracellular GSH content is reestablished.
As before mentioned also NO has a key role in skeletal muscle as it is involved in several functions including contractility and blood flow. Straightforward evidence of NO requirement in muscle physiology was obtained from experiments with NOSμ-/- animals, in whiche alterations in mitochondrial morphology, bioenergetics and unfolded protein response were demonstrated [62][50]. These modifications produced skeletal muscle dysregulation of growth and exercise performance. Generally, NO inhibits force production and modulate Ca2+ release by direct interaction with sGC and ryanodine receptors, respectively [63,64,65][51][52][53]. Moreover, it inhibits the activity of Ca2+-dependent sarcoplasmic reticulum (SR) ATPase to avoid the depletion of SR Ca2+ [66][54]. NO avidity to react with heme, the place where also oxygen binds, makes these two gaseous molecules in competition especially into the mitochondria, where high content of heme proteins and Fe-S groups are present to reduce the molecular oxygen concomitantly with ATP production. Indeed, the most recognized effect of NO on skeletal muscle metabolism is its capacity to directly inhibit mitochondrial electron transport chain complexes: NO inhibits electron transfer and NADH-dehydrogenase function at the level of Complex I through the intra-mitochondrial production of ONOO·− [67][55]; NO inhibits electron transfer at Complex III independently of oxygen concentration by inhibiting the transfer of electrons to cytochrome c and increasing the production of O2− [68][56]; NO inhibits cytochrome c oxidase activity in dependence of oxygen concentration and at the same time NO can be a good substrate for the enzyme. Then cytochrome c oxidase engagement with NO can be inhibitory of cellular respiration or for removal of NO from the cell [67,69][55][57]. At cellular level NO promotes muscle homeostasis by stimulating glucose transport during exercise trough activation of upstream signaling events leading to the translocation of glucose transporter GLUT4 at the cell surface [70][58]. One of the demonstrated mechanisms relies on the increase of cGMP level concomitantly with the activation of sGC during exercise. Chemical inhibition of NOS or nNOS knockout in mice muscle abolished such effects while NO donors raise cGMP level and glucose uptake [71][59].
NO modulates mitochondrial biogenesis through different routes, a selective one is by increasing the phosphorylated-active form of AMP-activated protein kinase (AMPK) that in turn induces PGC-1a signaling pathway [72][60]. PGC-1a is a nuclear and mitochondrial transcriptional coactivator inducing the expression of a large set of genes in response to metabolic and physiological stimuli, such as physical exercise [73,74][61][62]. Another pathway is shared with ROS by the activation of NFE2L2 expression, which is the master regulator of antioxidant response upon oxidative burst and mitochondrial biogenesis upon exercise in muscle [75][63]. NO modulates mitochondrial biogenesis also during myocytes differentiation by S-nitrosylation-mediated activation of the transcription factor CREB (cAMP response element-binding protein), which efficiently binds to the PGC-1a gene promoter region increasing its concentration and downstream signaling events [76][64]. Finally, NO produced by the endothelium affects skeletal muscle functions besides its canonical role in the regulation of vascular tone [77][65]. In fact, eNOS knockout mice (eNOS-/-) showed a reduction of mitochondrial complexes I and ATP synthase and altered skill in physical exercise: glucose and long-chain fatty acid uptake and glycogenolysis were markedly accelerated [78][66].
Through the formation of ONOO·− or ONOO·−-derived radical (NO2), NO is indirectly responsible for irreversible damage to protein residues such as tyrosine nitration. This modification can result in dramatic changes in the target protein structure/function with loss of the activity or acquisition of additional abnormal roles. Moreover, phosphorylative cascades can be altered being tyrosine a residue directly involved in phospho-mediated signaling pathways and the generation of antibodies against nitrated proteins induces immunological responses [79][67]. Protein carbonylation, which occurs on arginine, lysine, proline and threonine, represents the most widely studied marker for oxidative damage to proteins by high ROS levels; both carbonylated and nitrated proteins are accumulated in different types of muscle during aging or related pathologies [80,81,82][68][69][70] (Figure 3).
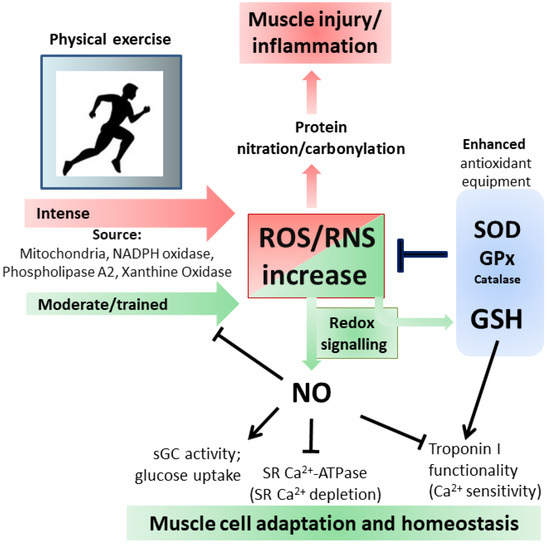
Figure 3. Oxidative and Nitrosative stress/signaling during intense and moderate physical exercise. For details see the text.
Considering the importance of GSH and NO in the control of muscle responsiveness, nutritional approaches aimed at increasing their concentrations have been tested to preserve muscle homeostasis. For instance, long-term supplementation with a cystine-based antioxidant compound is able to delay age-associated muscle loss [83][71]. Direct supplementation of GSH precursors, including N-acetylcysteine, L-alanine and L-glutamine, was also shown to promote muscle performance and recovery during exercise [84,85,86][72][73][74]. Nevertheless, the intake of antioxidants such as Vitamin C and E impedes a number of physiological responses activated during muscle training with no effect on GSH production [87,88][75][76]. This suggests that the promotion of GSH metabolism rather than blunting ROS production alone is the way for assuring adaptations to exercise [89][77]. On the other side, nutritional supplementation of the NO precursors L-Arginine and L-Citrulline has been clearly shown to ameliorate muscle homeostasis in terms of improved protein synthesis rates, myotube diameter and muscle antioxidant capacity [90,91][78][79]. These phenotypical advantages were also ascribed to the induction of iNOS expression and appreciable in wasting conditions obtained by deprivation of growth factors and/or nutrients.
References
- Lu, S.C. Regulation of Glutathione Synthesis. Mol. Asp. Med. 2009, 30, 42–59.
- Baldelli, S.; Aquilano, K.; Ciriolo, M.R. Punctum on two different transcription factors regulated by PGC-1α: Nuclear factor erythroid-derived 2-like 2 and nuclear respiratory factor 2. Biochim. Biophys. Acta 2013, 1830, 4137–4146.
- Lu, S.C. Regulation of hepatic glutathione synthesis: Current concepts and controversies. FASEB J. 1999, 13, 1169–1183.
- Xiong, Y.; Uys, J.D.; Tew, K.D.; Townsend, D.M. S-glutathionylation: From molecular mechanisms to health outcomes. Antioxid. Redox Signal. 2011, 15, 233–270.
- Aoyama, K.; Nakaki, T. Impaired glutathione synthesis in neurodegeneration. Int. J. Mol. Sci. 2013, 14, 21021–21044.
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell. Longev. 2013, 2013, 972913.
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009, 390, 191–214.
- Jackson, M.J.; McArdle, A. Age-related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. J. Physiol. 2011, 589, 2139–2145.
- Chen, G.; Wang, S.H.; Warner, T.D. Regulation of iNOS mRNA levels in endothelial cells by glutathione, a double-edged sword. Free Radic. Res. 2000, 32, 223–234.
- Zhou, X.J.; Vaziri, N.D.; Wang, X.Q.; Silva, F.G.; Laszik, Z. Nitric oxide synthase expression in hypertension induced by inhibition of glutathione synthase. J. Pharmacol. Exp. Ther. 2002, 300, 762–767.
- Aquilano, K.; Baldelli, S.; Cardaci, S.; Rotilio, G.; Ciriolo, M.R. Nitric oxide is the primary mediator of cytotoxicity induced by GSH depletion in neuronal cells. J. Cell. Sci. 2011, 124, 1043–1054.
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167.
- Moncada, S.; Higgs, E.A. Molecular mechanisms and therapeutic strategies related to nitric oxide. FASEB J. 1995, 9, 1319–1330.
- Grozdanovic, Z. NO message from muscle. Microsc. Res. Tech. 2001, 55, 148–153.
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259.
- Hussain, S.P.; He, P.; Subleski, J.; Hofseth, L.J.; Trivers, G.E.; Mechanic, L.; Hofseth, A.B.; Bernard, M.; Schwank, J.; Nguyen, G.; et al. Nitric Oxide Is a Key Component in Inflammation-Accelerated Tumorigenesis. Cancer Res. 2008, 68, 7130–7136.
- Hughes, M.N. Chemistry of nitric oxide and related species. Meth. Enzymol. 2008, 436, 3–19.
- Foster, M.W.; Hess, D.T.; Stamler, J.S. Protein S-nitrosylation in health and disease: A current perspective. Trends Mol. Med. 2009, 15, 391–404.
- Baldelli, S.; Aquilano, K.; Rotilio, G.; Ciriolo, M.R. Glutathione and copper, zinc superoxide dismutase are modulated by overexpression of neuronal nitric oxide synthase. Int. J. Biochem. Cell. Biol. 2008, 40, 2660–2670.
- Moellering, D.; Mc Andrew, J.; Patel, R.P.; Forman, H.J.; Mulcahy, R.T.; Jo, H.; Darley-Usmar, V.M. The induction of GSH synthesis by nanomolar concentrations of NO in endothelial cells: A role for gamma-glutamylcysteine synthetase and gamma-glutamyl transpeptidase. FEBS Lett. 1999, 448, 292–296.
- Filomeni, G.; Rotilio, G.; Ciriolo, M.R. Disulfide relays and phosphorylative cascades: Partners in redox-mediated signaling pathways. Cell. Death Differ. 2005, 12, 1555–1563.
- Gutteridge, J.M.C.; Halliwell, B. Mini-Review: Oxidative stress, redox stress or redox success? Biochem. Biophys. Res. Commun. 2018, 502, 183–186.
- Ridnour, L.A.; Thomas, D.D.; Mancardi, D.; Espey, M.G.; Miranda, K.M.; Paolocci, N.; Feelisch, M.; Fukuto, J.; Wink, D.A. The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxide species. Putting perspective on stressful biological situations. Biol. Chem. 2005, 385, 1–10.
- Stuehr, D.J.; Haque, M.M. Nitric oxide synthase enzymology in the 20 years after the Nobel Prize. Br. J. Pharmac. 2019, 176, 177–188.
- Förstermann, U.; Closs, E.I.; Pollock, J.S.; Nakane, M.; Schwarz, P.; Gath, I.; Kleinert, H. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension 1994, 23, 1121–1131.
- Larsson, B.; Phillips, S.C. Isolation and Characterization of a Novel, Human Neuronal Nitric Oxide Synthase cDNA. Biochem. Biophys. Res. Commun. 1998, 251, 898–902.
- Silvagno, F.; Xia, H.; Bredt, D.S. Neuronal Nitric-oxide Synthase-, an Alternatively Spliced Isoform Expressed in Differentiated Skeletal Muscle. J. Biol. Chem. 1996, 271, 11204–11208.
- McNeill, E.; Crabtree, M.J.; Sahgal, N.; Patel, J.; Chuaiphichai, S.; Iqbal, A.J.; Hale, A.B.; Greaves, D.R.; Channon, K.M. Regulation of iNOS function and cellular redox state by macrophage Gch1 reveals specific requirements for tetrahydrobiopterin in NRF2 activation. Free Rad. Biol. Med. 2015, 79, 206–216.
- Elfering, S.L.; Sarkela, T.M.; Giulivi, C. Biochemistry of Mitochondrial Nitric-oxide Synthase. J. Biol. Chem. 2002, 277, 38079–38086.
- Aguirre, E.; López-Bernardo, E.; Cadenas, S. Functional evidence for nitric oxide production by skeletal-muscle mitochondria from lipopolysaccharide-treated mice. Mitochondrion 2012, 12, 126–131.
- Stamler, J.S.; Meissner, G. Physiology of Nitric Oxide in Skeletal Muscle. Physiol. Rev. 2001, 81, 209–237.
- Villanueva, C.; Giulivi, C. Subcellular and cellular locations of nitric-oxide synthase isoforms as determinants of health and disease. Free Rad. Biol. Med. 2010, 49, 307.
- Bartesaghi, S.; Radi, R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018, 14, 618–625.
- Lück, G.; Hoch, W.; Hopf, C.; Blottner, D. Nitric Oxide Synthase (NOS-1) Coclustered With Agrin-Induced AChR-Specializations on Cultured Skeletal Myotubes. Mol. Cell. Neurosci. 2000, 16, 269–281.
- Loureiro, A.C.C.; do Rêgo-Monteiro, I.C.; Louzada, R.A.; Ortenzi, V.H.; de Aguiar, A.P.; de Abreu, E.S.; Cavalcanti-de-Albuquerque, J.P.A.; Hecht, F.; de Oliveira, A.C.; Ceccatto, V.M.; et al. Differential Expression of NADPH Oxidases Depends on Skeletal Muscle Fiber Type in Rats. Oxid. Med. Cell. Longev. 2016, 2016.
- Lee, S.; Shin, H.S.; Shireman, P.K.; Vasilaki, A.; Van Remmen, H.; Csete, M.E. Glutathione-peroxidase-1 null muscle progenitor cells are globally defective. Free Radic. Biol. Med. 2006, 41, 1174–1184.
- Ji, L.L.; Fu, R.; Mitchell, E.W. Glutathione and antioxidant enzymes in skeletal muscle: Effects of fiber type and exercise intensity. J. Appl. Physiol. 1992, 73, 1854–1859.
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276.
- Hellsten, Y.; Apple, F.S.; Sjödin, B. Effect of sprint cycle training on activities of antioxidant enzymes in human skeletal muscle. J. Appl. Physiol. 1996, 81, 1484–1487.
- Gnaiger, E. Capacity of oxidative phosphorylation in human skeletal muscle: New perspectives of mitochondrial physiology. Int. J. Biochem. Cell. Biol. 2009, 41, 1837–1845.
- Wang, P.; Li, C.G.; Qi, Z.; Cui, D.; Ding, S. Acute Exercise Induced Mitochondrial H2O2 Production in Mouse Skeletal Muscle: Association with p66Shc and FOXO3a Signaling and Antioxidant Enzymes. Available online: https://www.hindawi.com/journals/omcl/2015/536456/ (accessed on 6 September 2019).
- Leeuwenburgh, C.; Ji, L.L. Alteration of Glutathione and Antioxidant Status with Exercise in Unfed and Refed Rats. J. Nutr. 1996, 126, 1833–1843.
- Saoi, M.; Percival, M.; Nemr, C.; Li, A.; Gibala, M.; Britz-McKibbin, P. Characterization of the Human Skeletal Muscle Metabolome for Elucidating the Mechanisms of Bicarbonate Ingestion on Strenuous Interval Exercise. Anal. Chem. 2019, 91, 4709–4718.
- Steinbacher, P.; Eckl, P. Impact of Oxidative Stress on Exercising Skeletal Muscle. Biomolecules 2015, 5, 356–377.
- Pastore, A.; Piemonte, F. Protein Glutathionylation in Cardiovascular Diseases. Int. J. Mol. Sci. 2013, 14, 20845–20876.
- Alegre-Cebollada, J.; Kosuri, P.; Giganti, D.; Eckels, E.; Rivas-Pardo, J.A.; Hamdani, N.; Warren, C.M.; Solaro, R.J.; Linke, W.A.; Fernández, J.M. S-Glutathionylation of Cryptic Cysteines Enhances Titin Elasticity by Blocking Protein Folding. Cell 2014, 156, 1235–1246.
- Mollica, J.P.; Dutka, T.L.; Merry, T.L.; Lamboley, C.R.; McConell, G.K.; McKenna, M.J.; Murphy, R.M.; Lamb, G.D. S-Glutathionylation of troponin I (fast) increases contractile apparatus Ca2+ sensitivity in fast-twitch muscle fibres of rats and humans. J. Physiol. 2012, 590, 1443–1463.
- Dutka, T.L.; Mollica, J.P.; Lamboley, C.R.; Weerakkody, V.C.; Greening, D.W.; Posterino, G.S.; Murphy, R.M.; Lamb, G.D. S-nitrosylation and S-glutathionylation of Cys134 on troponin I have opposing competitive actions on Ca2+ sensitivity in rat fast-twitch muscle fibers. Am. J. Physiol. Cell. Physiol. 2016, 312, 316–327.
- Gill, R.M.; O’Brien, M.; Young, A.; Gardiner, D.; Mailloux, R.J. Protein S-glutathionylation lowers superoxide/hydrogen peroxide release from skeletal muscle mitochondria through modification of complex I and inhibition of pyruvate uptake. PLoS ONE 2018, 13, e0192801.
- De Palma, C.; Morisi, F.; Pambianco, S.; Assi, E.; Touvier, T.; Russo, S.; Perrotta, C.; Romanello, V.; Carnio, S.; Cappello, V.; et al. Deficient nitric oxide signalling impairs skeletal muscle growth and performance: Involvement of mitochondrial dysregulation. Skelet. Muscle 2014, 4, 22.
- Mészáros, L.G.; Minarovic, I.; Zahradnikova, A. Inhibition of the skeletal muscle ryanodine receptor calcium release channel by nitric oxide. FEBS Lett. 1996, 380, 49–52.
- Maréchal, G.; Gailly, P. Effects of nitric oxide on the contraction of skeletal muscle. Cell. Mol. Life Sci. 1999, 55, 1088–1102.
- Xiyuan, Z.; Fink, R.H.A.; Mosqueira, M. NO-sGC Pathway Modulates Ca2+ Release and Muscle Contraction in Zebrafish Skeletal Muscle. Front Physiol. 2017, 8, 607.
- Ishii, T.; Sunami, O.; Saitoh, N.; Nishio, H.; Takeuchi, T.; Hata, F. Inhibition of skeletal muscle sarcoplasmic reticulum Ca2+-ATPase by nitric oxide. FEBS Lett. 1998, 440, 218–222.
- Poderoso, J.J.; Carreras, M.C.; Lisdero, C.; Riobó, N.; Schöpfer, F.; Boveris, A. Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch. Biochem. Biophys. 1996, 328, 85–92.
- Iglesias, D.E.; Bombicino, S.S.; Valdez, L.B.; Boveris, A. Nitric oxide interacts with mitochondrial complex III producing antimycin-like effects. Free Radic. Biol. Med. 2015, 89, 602–613.
- Cooper, C.E.; Brown, G.C. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008, 40, 533–539.
- Lira, V.A.; Soltow, Q.A.; Long, J.H.D.; Betters, J.L.; Sellman, J.E.; Criswell, D.S. Nitric oxide increases GLUT4 expression and regulates AMPK signaling in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2007, 293, 1062–1068.
- Zhang, X.; Hiam, D.; Hong, Y.-H.; Zulli, A.; Hayes, A.; Rattigan, S.; McConell, G.K. Nitric oxide is required for the insulin sensitizing effects of contraction in mouse skeletal muscle. J. Physiol. 2017, 595, 7427–7439.
- Lira, V.A.; Brown, D.L.; Lira, A.K.; Kavazis, A.N.; Soltow, Q.A.; Zeanah, E.H.; Criswell, D.S. Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells. J. Physiol. 2010, 588, 3551–3566.
- Lira, V.A.; Benton, C.R.; Yan, Z.; Bonen, A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, 145–161.
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022.
- Merry, T.L.; Ristow, M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J. Physiol. 2016, 594, 5195–5207.
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Nuclear recruitment of neuronal nitric-oxide synthase by α-syntrophin is crucial for the induction of mitochondrial biogenesis. J. Biol. Chem. 2014, 289, 365–378.
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W.S. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94.
- Lee-Young, R.S.; Ayala, J.E.; Hunley, C.F.; James, F.D.; Bracy, D.P.; Kang, L.; Wasserman, D.H. Endothelial nitric oxide synthase is central to skeletal muscle metabolic regulation and enzymatic signaling during exercise in vivo. Am. J. Physiol. Regul. Integr. Comparat. Physiol. 2010, 298, 1399–1408.
- Batthyány, C.; Bartesaghi, S.; Mastrogiovanni, M.; Lima, A.; Demicheli, V.; Radi, R. Tyrosine-Nitrated Proteins: Proteomic and Bioanalytical Aspects. Antioxid. Redox Signal. 2017, 26, 313–328.
- Feng, J.; Navratil, M.; Thompson, L.V.; Arriaga, E.A. Principal component analysis reveals age-related and muscle-type-related differences in protein carbonyl profiles of muscle mitochondria. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 1277–1288.
- Murakami, H.; Guillet, C.; Tardif, N.; Salles, J.; Migné, C.; Boirie, Y.; Walrand, S. Cumulative 3-nitrotyrosine in specific muscle proteins is associated with muscle loss during aging. Exp. Gerontol. 2012, 47, 129–135.
- Cakatay, U.; Telci, A.; Kayali, R.; Tekeli, F.; Akçay, T.; Sivas, A. Relation of aging with oxidative protein damage parameters in the rat skeletal muscle. Clin. Biochem. 2003, 36, 51–55.
- Sinha-Hikim, I.; Sinha-Hikim, A.P.; Parveen, M.; Shen, R.; Goswami, R.; Tran, P.; Crum, A.; Norris, K.C. Long-term supplementation with a cystine-based antioxidant delays loss of muscle mass in aging. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 749–759.
- Petry, E.R.; Cruzat, V.F.; Heck, T.G.; Leite, J.S.M.; Homem de Bittencourt, P.I.; Tirapegui, J. Alanyl-glutamine and glutamine plus alanine supplements improve skeletal redox status in trained rats: Involvement of heat shock protein pathways. Life Sci. 2014, 94, 130–136.
- Leite, J.S.M.; Raizel, R.; Hypólito, T.M.; Rosa, T.D.S.; Cruzat, V.F.; Tirapegui, J. l-glutamine and l-alanine supplementation increase glutamine-glutathione axis and muscle HSP-27 in rats trained using a progressive high-intensity resistance exercise. Appl. Physiol. Nutr. Metab. 2016, 41, 842–849.
- Michailidis, Y.; Karagounis, L.G.; Terzis, G.; Jamurtas, A.Z.; Spengos, K.; Tsoukas, D.; Chatzinikolaou, A.; Mandalidis, D.; Stefanetti, R.J.; Papassotiriou, I.; et al. Thiol-based antioxidant supplementation alters human skeletal muscle signaling and attenuates its inflammatory response and recovery after intense eccentric exercise. Am. J. Clin. Nutr. 2013, 98, 233–245.
- Venditti, P.; Napolitano, G.; Barone, D.; Di Meo, S. Vitamin E supplementation modifies adaptive responses to training in rat skeletal muscle. Free Radic. Res. 2014, 48, 1179–1189.
- Morrison, D.; Hughes, J.; Della Gatta, P.A.; Mason, S.; Lamon, S.; Russell, A.P.; Wadley, G.D. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic. Biol. Med. 2015, 89, 852–862.
- Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Cabo, H.; Ferrando, B.; Viña, J. Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training? Free Radic. Biol. Med. 2015, 86, 37–46.
- Wang, R.; Jiao, H.; Zhao, J.; Wang, X.; Lin, H. L-Arginine Enhances Protein Synthesis by Phosphorylating mTOR (Thr 2446) in a Nitric Oxide-Dependent Manner in C2C12 Cells. Oxid. Med. Cell. Longev. 2018, 2018, 7569127.
- Ham, D.J.; Gleeson, B.G.; Chee, A.; Baum, D.M.; Caldow, M.K.; Lynch, G.S.; Koopman, R. L-Citrulline Protects Skeletal Muscle Cells from Cachectic Stimuli through an iNOS-Dependent Mechanism. PLoS ONE 2015, 10, e0141572.
More
