The genus Diabrotica has over 400 described species, the majority of them neotropical. However, only three species of neotropical Diabrotica are considered agricultural pests, D. speciosa, D. balteata, and D. viridula. D. speciosa and D. balteata are polyphagous both as adults and during the larval stage. D. viridula are stenophagous during the larval stage, feeding essentially on maize roots, and polyphagous as adults. The larvae of the three species are pests on maize, but D. speciosa larvae also feed on potatoes and peanuts, while D. balteata larvae feed on beans and peanuts. None of these species express a winter/dry season egg diapause, like several North American species. Instead they have several continuous, latitude-mediated generations per year. This hinders the use of crop rotation as a management tool, although early planting can help in the temperate regions of the distribution of D. speciosa. Their know parasitoids do not exert much control on Diabrotica populations, or show potential for inundative biocontrol plans. Management options are limited to insecticide applications and Bt GM maize. Other techniques that show promise are products using Beauveria bassiana and Heterorhabditis bacteriophora, semiochemical attractants for monitoring purposes or as toxic baits, and plant resistance.
- Diabrotica speciosa
- Diabrotica balteata
- Diabrotica viridula
- rootworm management
- maize pests
- cucurbitacins
- semiochemicals
1. Definition
There a three pest Diabrotica in South America from over 400 described species in the genus, the majority of them neotropical.
2. General Biology of South American Pest Diabrotica
The genus
Diabroticahas over 400 described species [1], the majority of them neotropical, but only 7 species, plus six subspecies, are considered agricultural pests in the Americas [2]. Of these, only three species are considered agricultural pests in South America:
D. speciosa(Germar) with subspecies
speciosaand
vigens,
D. balteata(LeConte), and
D. viridula(F.) (
Figure 1). The genus
Diabroticais divided into three species groups:
virgifera,
fucata, and
signifera [3,4]. However, studies on South American[3][4]. However, studies on South American
virgifera group species suggest that these groups are not as well defined as previously thought [5,6].group species suggest that these groups are not as well defined as previously thought [5][6].
D. speciosaand
D. balteataare in the
fucatagroup, which is the group with the largest number of species. The species in this group that have been studied are polyphagous both as adults and during the larval stage. Another characteristic of the North American pest
Diabroticaof the
fucataspecies group is that they overwinter as adults and lack resistant stages to deal with harsh climatic conditions [2].
D. viridulais in the
virgiferagroup, the same clade of the Northern, Western, and Mexican corn rootworms (
Diabrotica barberi,
Diabrotica virgifera virgifera, and
Diabrotica virgifera zeae, respectively). The larvae of the North American species in the
virgiferagroup feed exclusively on Poaceae [7], although the host range has been observed or tested for only a few of the species in the group [8]. The North American pest species in the
virgifera species group are univoltine, or sometimes semivoltine, and possess diapausing eggs that allow them to overwinter in temperate climates or survive dry seasons in the subtropics [9,10], both situations during which the adult cannot find sustenance or survive the extreme conditions.species group are univoltine, or sometimes semivoltine, and possess diapausing eggs that allow them to overwinter in temperate climates or survive dry seasons in the subtropics [9][10], both situations during which the adult cannot find sustenance or survive the extreme conditions.

Photographs of the adult of the three species of pest
Diabrotica from South America.from South America.
is distributed throughout South America, from agricultural patches in the temperate Patagonian steppes to the tropics, with the exception of Chile, and up to altitudes of over 2500 m above sea level [2][11] (
Figure 2). It is the best studied
Diabroticaspecies in South America due to its impact on many crops. The adult has over 132 recorded host species, in 24 different plant families [11, and literature therein]. Larval hosts are not as well known, but
D. speciosahas at least five confirmed larval hosts: maize (
Zea maysL.), wheat (
Triticumspp.), Johnsongrass (
Sorghum halepensePersoon), peanut (
Arachis hypogaeaL.), and potato (
Solanum tuberosum L.). Another four plant species hosted full development in the laboratory [11,12,13,14,15]. However, the fact that larvae can develop on plant species in four families of three different orders suggests that there could be many more larval hosts that simply have not been discovered because of the hypogeous habit of the larva.L.). Another four plant species hosted full development in the laboratory [11][12][13][14][15]. However, the fact that larvae can develop on plant species in four families of three different orders suggests that there could be many more larval hosts that simply have not been discovered because of the hypogeous habit of the larva.
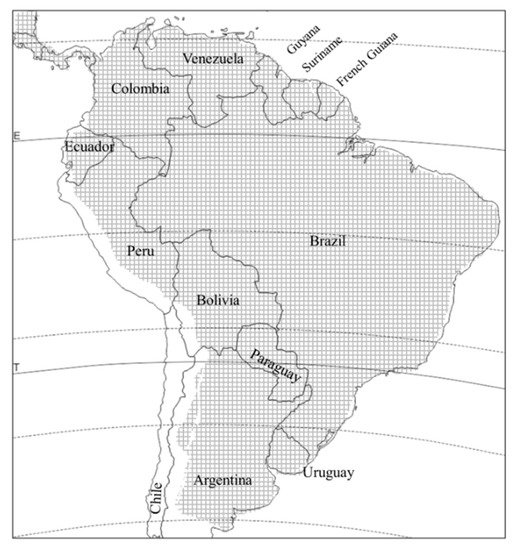
Distribution of
Diabrotica speciosa in South America (crosshatched area).in South America (crosshatched area).
is documented in most crops in South America, but is considered mainly a horticultural pest as an adult, and a pest of potato, maize, and peanuts as larva [11][13][16]. Yet these generalizations are not without exceptions. In Brazil, this species is considered a pest of maize as a larva, and a minor pest as an adult as well [17][18]. It is also regarded as an important pest of potato during both the adult and larval stages, although this depends heavily on the cultivar [19]. In addition, the adult is also regarded as an important pest of seedlings and young plants of some extensive crops, such as soybeans, beans (
Phaseolus vulgaris), cotton, sunflower, maize, tobacco, wheat, and canola [20,21,22], and, curiously, of table grapes [23] (), cotton, sunflower, maize, tobacco, wheat, and canola [20][21][22], and, curiously, of table grapes [23] (
Table 1).).
Main crops attacked by the South American pest
Diabrotica species, and current and potential control methods.species, and current and potential control methods.
| D. balteata | D. speciosa | D. viridula | Control Methods | Promising Control Methods | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Host Crop | Adults | Larvae | Adults | Larvae | Adults | Larvae | Adults | Larvae | Adults | Larvae |
| beans | x | x | x | x | Cb, Op, Nn, Py 1 | intercropping plant resistance |
||||
| cucurbits | x | x | Cb, Op, Nn, Py | cucurbitacin baits | ||||||
| maize | x | x | x | x | Cb, Op, Nn, Py | Bt maize seed treatment (Nn, Cb, Di) 1 |
silicon cucurbitacin baits |
IGR 1 seed treatment with fungi, plant resistance, nematodes |
||
| peanuts | x | x | x | Cb, Op, Nn, Py | ||||||
| potatoes | x | x | x | Nn | plant resistance | plant resistance, nematodes |
||||
| soybeans | x | Cb, Op, Nn, Py | ||||||||
| tobacco | x | Cb, Op, Nn, Py | ||||||||
is found from subtropical North America through Central America and Caribbean islands including Cuba, Hispaniola, and Puerto Rico, to South America, although its distribution in South America is limited to Venezuela and Colombia [2][24], where it can occur at altitudes ranging from 0 to 2000 m [25]. However, there is insufficient data to infer species distribution patterns in either country. The adult of
D. balteataalso has an extremely wide range of host plants, as it has been documented on over 140 plant species [26]. There is a more conservative estimate of 50 species in 23 families, with a preference for plants in the Cucurbitaceae, Rosaceae, Fabaceae, and Brassicaceae [27]. The
D. balteataadult is considered a pest on squash (
Cucurbitaspp., Cucurbitaceae), several bean species (
P. vulgaris,
Glycine max,
Mucuna pruriens, and
Vigna unguiculata, Fabaceae), lettuce (
Lactuca sativa, Asteraceae), sugar cane (
Saccharum officinarum, Poaceae), and potato [28]. Adults are also implicated in the transmission of the tomato brown rugose fruit virus (Tobamovirus, ToBRFV) to
P. vulgaris[29], and other viruses of
P. vulgarisand calapo (
Calopogonium mucunoides Desv.) [30,31]. Larval damage is reported only from Colombia, where this species is known to attack beans, but as considered a minor problem [32], maize, on which it can be locally problematic [33,34], and peanuts, on which it is considered among the 10–12 worst pests in Colombia [35] (Desv.) [30][31]. Larval damage is reported only from Colombia, where this species is known to attack beans, but as considered a minor problem [32], maize, on which it can be locally problematic [33][34], and peanuts, on which it is considered among the 10–12 worst pests in Colombia [35] (
Table 1). It is considered a pest of sweetpotatoes in North America. The fact that these hosts are also from three families in three orders suggests that there could be many more larval hosts as well. In addition, phylogenetic studies indicate
D. speciosaand
D. balteata are sister clades [37].are sister clades [36].
is distributed from Mexico to northern Argentina, and apparently absent in Uruguay and Chile (
Figure 3). Like
D. balteata, its distribution is primarily tropical and subtropical. The
D. viridula adult is considered a minor pest of beans in Peru [39], while the larva is considered locally important on maize in Central America and Peru [40]. In greenhouse tests, both the larvae and the adults of this species were able to transmit maize chlorotic mottle virus (MCMV) to maize, and they are assumed to be one of its vectors in the field [41].adult is considered a minor pest of beans in Peru [37], while the larva is considered locally important on maize in Central America and Peru [38]. In greenhouse tests, both the larvae and the adults of this species were able to transmit maize chlorotic mottle virus (MCMV) to maize, and they are assumed to be one of its vectors in the field [39].
D. viridula is also assumed to be an important, albeit new, pest of maize roots in Argentina, Paraguay, and Brazil [11,42], but its damage cannot be differentiated from that ofis also assumed to be an important, albeit new, pest of maize roots in Argentina, Paraguay, and Brazil [11][40], but its damage cannot be differentiated from that of
D. speciosa. Studies to clarify what proportion of the damage is owed to each species (e.g., collections of larvae directly in the field) have not been done. The larva has been found feeding on maize roots only, and in the laboratory, it developed successfully on wheat as well, but not on any of the species tested from outside the Poaceae, suggesting it is stenophagous during the larval stage [2,43]. As an adult it is polyphagous, albeit reduced to fewer hosts than. Studies to clarify what proportion of the damage is owed to each species (e.g., collections of larvae directly in the field) have not been done. The larva has been found feeding on maize roots only, and in the laboratory, it developed successfully on wheat as well, but not on any of the species tested from outside the Poaceae, suggesting it is stenophagous during the larval stage [2][41]. As an adult it is polyphagous, albeit reduced to fewer hosts than
D. speciosaand
D. balteata, as it has been recorded only on 21 plant species in the Poacae, Cucurbitaceae, and Asteraceae [11] (, as it has been recorded only on 21 plant species in the Poacae, Cucurbitaceae, and Asteraceae [11] (
Table 1). Yet, similarities with the North American species in the
virgiferagroup end here, as
D. viridula eggs do not diapause.eggs do not diapause.
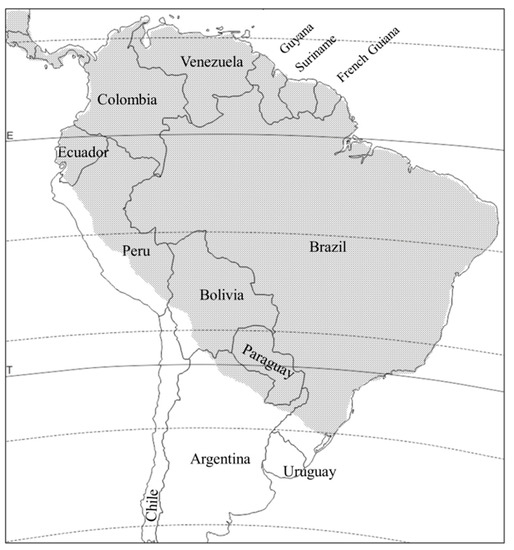
Distribution of
in South America (stippled area).
Evidence indicates that the three South American pest
Diabroticaoverwinter as adults, are multivoltine, and do not have diapausing eggs. A reproductive diapause has been observed for
D. speciosa, at least for the populations from the temperate and higher subtropical areas, but the fact that it could be overridden by manipulating temperature and light hours suggests it may not exist in the lower latitudes [44]., at least for the populations from the temperate and higher subtropical areas, but the fact that it could be overridden by manipulating temperature and light hours suggests it may not exist in the lower latitudes [42].
3. Control of South American
Diabrotica
As the North American corn rootworms in the
virgifera species group overwinter as diapausing eggs, are univoltine, and have a narrow larval host range limited to maize and a few grasses, their life cycle is tightly coupled to the phenology of one or very few annual host species. This provides opportunities for the use of different management strategies to reduce damage levels on susceptible crops, such as crop rotation and manipulation of sowing dates [45,46], expected density functions based on preceding density data [47], and anticipation of adult appearance through degree-day models [48]. Also, as the eggs are found anywhere in the soil from before the crop is planted, different tillage techniques could be applied to hinder the larvae from reaching the roots, for instance, compacting the soil between rows, thus affecting neonate larval movement [49]. Furthermore, factors behind the recommencement and completion of embryonic development after winter in univoltinespecies group overwinter as diapausing eggs, are univoltine, and have a narrow larval host range limited to maize and a few grasses, their life cycle is tightly coupled to the phenology of one or very few annual host species. This provides opportunities for the use of different management strategies to reduce damage levels on susceptible crops, such as crop rotation and manipulation of sowing dates [43][44], expected density functions based on preceding density data [45], and anticipation of adult appearance through degree-day models [46]. Also, as the eggs are found anywhere in the soil from before the crop is planted, different tillage techniques could be applied to hinder the larvae from reaching the roots, for instance, compacting the soil between rows, thus affecting neonate larval movement [47]. Furthermore, factors behind the recommencement and completion of embryonic development after winter in univoltine
Diabrotica are fairly well understood, so it is possible to estimate a “fixed point” (or interval) for the conclusion of embryonic development of the egg bank laid during the previous season in any given area [50]. However, none of these options have been developed for multivoltine species.are fairly well understood, so it is possible to estimate a “fixed point” (or interval) for the conclusion of embryonic development of the egg bank laid during the previous season in any given area [48]. However, none of these options have been developed for multivoltine species.
The field biology of the multivoltine species of the North American pest
Diabrotica is also relatively well understood. Yet, in contrast to the univoltine species, predicting the incidence of the multivoltine species is not easily achieved.is also relatively well understood. Yet, in contrast to the univoltine species, predicting the incidence of the multivoltine species is not easily achieved.
Although it is certain that the South American pest
Diabrotica are multivoltine, seasonal reproductive patterns are not well known for these species, yet different models indicate there could be around three generations a year, and in subtropical regions, no fewer than five. However, no obvious or discrete voltinism pattern could be observed, expressing, to all practical effects, continuous generations [53]. What is known of the reproductive biology of the other two pest species suggests the same may be expected for them. The practical implications of these studies were that the life history pattern of this pest seems to leave few management alternatives. In the temperate regions of this species’ distribution, early planting of maize could ensure that the first generations of larvae encounter more mature, and thus less susceptible stages of the crop. Other than this, the seasonal dispersion and unpredictability ofare multivoltine, seasonal reproductive patterns are not well known for these species, yet different models indicate there could be around three generations a year, and in subtropical regions, no fewer than five. However, no obvious or discrete voltinism pattern could be observed, expressing, to all practical effects, continuous generations [49]. What is known of the reproductive biology of the other two pest species suggests the same may be expected for them. The practical implications of these studies were that the life history pattern of this pest seems to leave few management alternatives. In the temperate regions of this species’ distribution, early planting of maize could ensure that the first generations of larvae encounter more mature, and thus less susceptible stages of the crop. Other than this, the seasonal dispersion and unpredictability of
D. speciosa outbreaks suggest that the only pre-emptive action available to protect maize crops from this pest is to plant Bt maize [53].outbreaks suggest that the only pre-emptive action available to protect maize crops from this pest is to plant Bt maize [49].
As mentioned above, the damage on maize from
D. speciosalarval feeding cannot be differentiated from that of
D. viridula, so control measures implemented for the control of
D. speciosalarvae apply to
D. viridulaas well (
Figure 4).).

Top, typical damage on maize roots and lodging caused by
D. speciosaand
D. viridulalarvae. (photos by Dirceu N. Gassen); below,
D. speciosa larva on potato with typical pinprick damage (photo by Pablo Lanzetta).larva on potato with typical pinprick damage (photo by Pablo Lanzetta).
4. Conclusions
Apart from insecticide applications, the major innovation of applicable use of the last 30 years has been the introduction of GM maize. However, other techniques that show promise must continue to be explored, such as the use of toxic baits with semiochemical attractants to suppress adult populations and for monitoring purposes, IGR insecticides aimed at adults to reduce their progeny, development of plant resistance, and biological control using
Heterorhabditis nematodes and entomopathogenic fungus against larvae. Insecticide + cucurbitacin baits also deserve a special mention, because this combination has proved to be an effective technique that probably warrants further development.nematodes and entomopathogenic fungus against larvae. Insecticide + cucurbitacin baits also deserve a special mention, because this combination has proved to be an effective technique that probably warrants further development.
Pest
Diabroticain South America are widely regarded as important, but usually are not differentiated from other foliar pests or root-feeders when it comes to management. Farmers do not identify them among the worst pests, and seldom deploy specific control measures for these beetles, except for potatoes in Brazil, where producers consider
D. speciosato be the main pest. Yet, the actual impact of the larvae of
D. speciosaand
D. viridula, especially on maize, may not be properly assessed, and until that is done, we cannot be sure of the real importance of these pests., especially on maize, may not be properly assessed, and until that is done, we cannot be sure of the real importance of these pests.
References
- Derunkov, A.; Konstantinov, A. Taxonomic changes in the genus Diabrotica Chevrolat (Coleoptera: Chrysomelidae: Galerucinae): Results of a synopsis of North and Central America Diabrotica species. Zootaxa 2013, 3686, 301–325.
- Krysan, J.L. Introduction: Biology, distribution, and identification of pest Diabrotica. In Methods for the Study of Pest Diabrotica, 1st ed.; Krysan, J.L., Miller, T.A., Eds.; Springer: New York, NY, USA, 1986; pp. 1–23.
- Wilcox, J.A. Chrysomelidae: Galerucinae: Luperini: Diabroticina; Pars. 78, Fasc. 2. In Coleopterum Catalogus Supplementa, 1st ed.; Wilcox, J.A., Ed.; Uitgeverij Dr. W. Junk’s: Gravenhage, The Netherlands, 1972; pp. 296–343.
- Krysan, J.L.; Smith, R.F. Systematics of the virgifera species group of Diabrotica (Coleoptera: Chrysomelidae: Galerucinae). Entomography 1987, 5, 375–484.
- Cabrera, N.; Sosa Gómez, D.; Micheli, A. A morphological and molecular characterization of a new species of Diabrotica (Coeloptera: Chrysomelidae: Galerucinae). Zootaxa 2008, 1922, 33–46.
- Cabrera, N.; Cabrera Walsh, G. Diabrotica collicola (Coleoptera: Chrysomelidae), a new species of leaf beetle from Argentina. Discussion and key to some similar species of the Diabrotica virgifera group. Zootaxa 2010, 2683, 45–55.
- Branson, T.F.; Krysan, J.L. Feeding and oviposition behavior and life cycle strategies of Diabrotica: An evolutionary view with implications for pest management. Environ. Entomol. 1981, 10, 826–831.
- Clark, T.L.; Hibbard, B.E. Comparison of nonmaize hosts to support western corn rootworm (Coleoptera: Chrysomelidae) larval biology. Environ. Entomol. 2004, 33, 681–689.
- Krysan, J.L. Diapause in the neartic species of the virgifera group of Diabrotica: Evidence for tropical origin and temperate adaptations. Ann. Entomol. Soc. Am. 1982, 75, 136–142.
- Krysan, J.L.; Branson, T.F.; Díaz Castro, G. Diapause in Diabrotica virgifera (Coleoptera: Chrysomelidae): A comparison of eggs from temperate and subtropical climates. Entomol. Exp. Appl. 1977, 22, 81–89.
- Cabrera Walsh, G.; Cabrera, N. Distribution and hosts of the pestiferous and other common Diabroticites from Argentina and Southern South America: A geographic and systematic view. In New Developments in the Biology of Chrysomelidae; Jolivet, P.H., Santiago-Blay, J.A., Schmitt, M., Eds.; SPB Academic Publishers: The Hague, The Netherlands, 2004; pp. 333–350.
- Ávila, C.J.; Parra, J.R.P. Desenvolvimento de Diabrotica speciosa (Germar) (Coleoptera: Chrysomelidae) em diferentes hospedeiros. Cienc. Rural 2002, 32, 739–743.
- Cabrera Walsh, G. Host range and reproductive traits of Diabrotica speciosa (Germar) and Diabrotica viridula (F.) (Coleoptera: Chrysomelidae), two species of South American pest rootworms, with notes on other species of Diabroticina. Environ. Entomol. 2003, 32, 276–285.
- Cabrera Walsh, G. Sorghum halepense (L.) Persoon (Poaceae), a new larval host for the South American corn rootworm Diabrotica speciosa (Germar) (Coleoptera: Chrysomelidae). Coleopt. Bull. 2007, 61, 83–84.
- Ávila, C.J.; Bitencourt, D.R.; Silva, I.F. Biology, reproductive capacity, and foliar consumption of Diabrotica speciosa (Germar) (Coleoptera: Chrysomelidae) in different host plants. J. Agric. Sci. 2019, 11, 1–9.
- Marques, G.B.C.; Ávila, C.J.; Parra, J.R.P. Danos causados por larvas e adultos de Diabrotica speciosa (Coleoptera: Chrysomelidae) em milho. Pesqui. Agropecu. Bras. 1999, 34, 1983–1986.
- Gassen, D.N. Insetos Subterráneos Perjudiciais às Culturas no Sul de Brasil Documentos, 13; Embrapa-CNPT: Passo Fundo, Brazil, 1989; pp. 32–33.
- Ávila, C.J.; Milanez, J.M. Larva alfinete. In Pragas de Solo no Brasil; Salvadori, J.R., Ávila, C.J., Silva, M.T.B., Eds.; Fundacep-Fecotrigo: Passo Fundo/Dourados/Cruz Alta, Brazil, 2004; pp. 345–378.
- Salles, L.A. Incidência de danos de Diabrotica speciosa en cultivares e linhagens de batata. Cienc. Rural 2000, 30, 205–209.
- Haji, N.F.P. Biologia, dano e controle do adulto de Diabrotica speciosa (Germar, 1824) (Coleoptera: Chrysomelidae na cultura da batatinha (Solanum tuberosum L.). Ph.D. Thesis, Escola Superior de Agricultura “Luiz de Queiroz”, Piracicaba, Brazil, 1981.
- Ávila, C.J. Principais pragas e seu controle. In A Cultura do Feijoeiro em Mato Grosso do Sul, Circular Tecnica 17; Embrapa-UEPAE: Dourados, Brazil, 1990; pp. 54–56.
- Ávila, C.J.; Santana, A.G. Cap. 4: Danos causados às culturas por adultos e larvas de Diabrotica speciosa. In Diabrotica speciosa, 1st ed.; Nava, D.E., Ávila, C.J., Pinto, A.S., Eds.; Occasio Editora: Piracicaba/São Paulo, Brasil, 2016; pp. 59–67.
- Roberto, S.R.; Genta, W.; Ventura, M.U. Diabrotica speciosa (Ger.) (Coleoptera: Chrysomelidae): New pest in table grape orchards. Neotrop. Entomol. 2001, 30, 721–722.
- Segarra-Carmona, A.E.; Flores-López, L.; Cabrera-Asencio, I. New report of a leaf beetle pest from North America in Puerto Rico: Diabrotica balteata Le Conte (Coleoptera: Chrysomelidae) and its chemical control. J. Agric. Univ. Puerto Rico 2008, 92, 119–122.
- Gonzalez, R.; Cardona, C.; Schoonhoven, A.V. Morfología y biología de los crisomélidos Diabrotica balteata LeConte y Cerotoma facialis Erickson como plagas del frijol común. Turrialba 1982, 32, 257–264.
- Clark, S.M.; LeDoux, D.G.; Seeno, T.N.; Riley, E.G.; Gilbert, A.J.; Sullivan, J.M. Host Plants of Leaf Beetle Species Occurring in the United States and Canada (Coleoptera: Megalopodidae, Orsodacnidae, Chrysomelidae, Excluding Bruchinae), Special Publication No. 2; Coleopterists Society: Sacramento, CA, USA, 2004; pp. 86–87.
- Saba, F. Host plant spectrum and temperature limitations of Diabrotica balteata. Can. Entomol. 1970, 102, 684–691.
- Agrosavia. Available online: https://www.agrosavia.co/ctni/ctc/coleoptera/chrysomelidae/diabrotica/diabrotica-balteata (accessed on 16 April 2020).
- Morales, F.; Gámez, R. Beetle-transmitted viruses. In Bean Production Problems in the Tropics, 2nd ed.; Schwartz, H.F., Pastor Corrales, M.A., Eds.; CIAT: Cali, Colombia, 1989; pp. 363–378.
- Cano Piedrahíta, C.A. Evaluación de tres Extractos Vegetales para el Control de Plagas en el Cultivo de Frijol Arbustivo Phaseolus vulgaris L. Master’s Thesis, Universidad de Manizales, Caldas, Colombia, 2016.
- Morales, F.J.; Castano, M.; Arroyave, J.A.; Ospina, M.D.; Calvert, L.A. A sobemovirus hindering the utilization of Calopogonium mucunoides as a forage legume in the lowland tropics. Plant Dis. 1995, 79, 1220–1224.
- Cardona, C.; Gonzalez, R.; Schoonhoven, A.V. Evaluation of damage to common beans by larvae and adults of Diabrotica balteata and Cerotoma facialis. J. Econ. Entomol. 1982, 75, 324–327.
- Bandas, L.D.C.; Corredor, D.; Corredor, S. Efecto de la asociación patilla (Citrullus lanatus) con maíz (Zea mays) sobre la población y daño causado por tres insectos plaga y el rendimiento de estos cultivos en la Ciénaga Grande de Lorica, Córdoba. Rev. Colomb. Entomol. 2004, 30, 161–169.
- Rodríguez Chalarca, J.; Valencia, S.J. Daño por larvas de Diabrotica balteata (Coleoptera: Chrysomelidae) en raíces de maíz en condiciones controladas. In Proceedings of the 39 Congreso de la Sociedad Colombiana de Entomología, Ibagué, Universidad Cooperativa de Colombia, Bogota, Colombia, 11–13 June 2012; p. 93.
- Tobar, J.A. Manejo Integrado de Insectos Plaga en el Cultivo de la Mani (Arachis hypogaea L.); Facultad de Ciencias Agrícolas, Universidad de Nariño: Nariño, Colombia, 1990; p. 21.
- Clark, T.L.; Meinke, L.J.; Foster, J.E. Molecular phylogeny of Diabrotica beetles (Coleoptera: Chrysomelidae) inferred from analysis of combined mitochondrial and nuclear DNA sequences. Insect Mol. Biol. 2001, 10, 303–314.
- Anteparra, M.; Velásquez, J. Revisión de la familia Chrysomelidae asociada a leguminosas de grano en el trópico sudamericano. Invest. Amazonía 2015, 4, 62–69.
- King, A.B.S.; Saunders, J.L. The Invertebrate Pests of Annual Food Crops in Central America, 1st ed.; Overseas Development Administration: London, UK, 1984; pp. 44–45.
- Reyes, H.E.; Castillo, L.J. Transmisión del virus del moteado clorótico del maíz (maize chlorotic mottle virus -MCMV) por dos especies del género Diabrotica, familia Chrysomelidae. Fitopatología 1988, 23, 65–73.
- Waquil, J.M.; Mendes, S.M.; Marucci, R.C. Comunicado Técnico 178: Ocorrência de Especies de Diabrotica em milho no Brasil: Qual a Predominante, Diabrotica Speciosa ou Diabrotica Viridula; Embrapa Milho e Sorgo: Sete Lagoas/Minas Gerais, Brazil, 2010; pp. 1–6.
- Cabrera Walsh, G. Laboratory rearing and vital statistics of Diabrotica speciosa (Germar) and Diabrotica viridula (F.) (Coleoptera: Chrysomelidae), two species of South American pest rootworms. Rev. Soc. Entomol. Argent. 2001, 60, 239–248.
- Cabrera Walsh, G. Crisomélidos Diabroticinos Americanos: Hospederos y Enemigos Naturales. Biología y Factibilidad de Manejo de las Especies Plaga, 1st ed.; Lap Lambert Academic Publishing GmbH & Co.: Saarbrücken, Germany, 2012; pp. 42–60.
- Levine, E.; Oloumi-Sadeghi, H. Management of diabroticite rootworms in corn. Annu. Rev. Entomol. 1991, 36, 229–255.
- Spencer, J.L.; Hibbard, B.E.; Moeser, J.; Onstad, D.W. Behaviour and ecology of the western corn rootworm (Diabrotica virgifera virgifera LeConte). Agric. For. Entomol. 2009, 11, 9–27.
- Schaafsma, A.W.; Whitfield, G.H.; Ellis, C.R. A temperature-dependent model of egg development of the western corn rootworm, Diabrotica virgifera virgifera Leconte (Coleoptera: Chrysomelidae). Can. Entomol. 1991, 123, 1183–1197.
- Stevenson, D.E.; Michels, G.J.; Bible, J.B.; Jackman, J.A.; Harris, M.K. Physiological time model for predicting adult emergence of western corn rootworm (Coleoptera: Chrysomelidae) in the Texas High Plains. J. Econ. Entomol. 2008, 101, 1584–1593.
- Park, Y.; Tollefson, J.J. Spatial prediction of corn rootworm (Coleoptera: Chrysomelidae) adult emergence in Iowa cornfields. J. Econ. Entomol. 2005, 98, 121–128.
- Meinke, L.J.; Sappington, T.W.; Onstad, D.W.; Guillemaud, T.; Miller, N.J.; Komáromi, J.; Levay, N.; Furlan, L.; Kiss, J.; Toth, F. Western corn rootworm (Diabrotica virgifera virgifera LeConte) population dynamics. Agric. For. Entomol. 2009, 11, 29–46.
- Cabrera Walsh, G.; Sacco, J.; Mattioli, F. Voltinism of Diabrotica speciosa (Coleoptera: Chrysomelidae) in Argentina: Latitudinal clines and implications for damage anticipation. Pest Manag. Sci. 2013, 69, 1272–1279.
