Molecularly imprinted polymers (MIPs) are versatile materials that mimic natural antigen–antibody mechanisms and allow molecules/analytes recognition [2,3]. MIPs have been used as selective sorbents for (micro)solid extraction (µ-SPE) procedures leading to molecularly imprinted (micro)solid extraction (MIMSPE), which allows advanced miniaturized sample pre-treatments for green procedures in Analytical Chemistry.
- molecularly imprinted polymers
- magnetic molecularly imprinted polymers
- dispersive (micro)solid phase extraction
1. Introduction
2. Dispersive (Micro)Solid Phase Extraction with MIPs
As shown in Figure 1, dSPE and D-µ-SPE [9,10,11,12][3][4][5][6] procedures consist of dispersing the adsorbent (a few milligrams or a very few milligrams) into the sample/extract by shaking (oscillators and vortex) and by applying ultrasounds, and, for magnetic adsorbents, by magnetic stirring [4][7]. Dispersion enhances target adsorption on the adsorbent (nano)microparticles, and the use of ultrasound and mechanical shaking (mainly vortex) favors adsorbent dis-aggregation and maximizes the surface area of the adsorbent particles. Vortex stirring is a soft and low-cost shaking technique and dispersion assistance is more repeatable when compared with ultrasounds because of the ultrasound fluency dependence on the position inside the water-bath tank [11][5]. Vortex assistance also prevents analyte degradation and adsorbent aggregation, although the technique offers lower extraction kinetics when compared to ultrasounds dispersion [13,14,15][8][9][10] (in fact, some reports have stated that ultrasounds change the absorption kinetics [16,17,18][11][12][13]).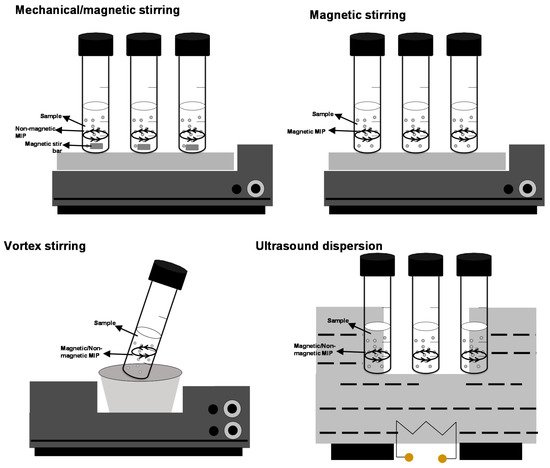
2.1. Dispersive (Micro)Solid Phase Extraction with Magnetic Molecularly Imprinted Polymers (MMIPs)
MMIP beads were first introduced by Ansell and Mosbach in 1998 as a core–shell structure (magnetic iron oxide, magnetite, Fe3O4) for performing drug radioligand binding assays [19][14]. Then, MMIPs (magnetic nickel hexacyanoferrate, NiHCF, nanoparticles coated with a molecularly imprinted polymer for the herbicide chlorotoluron) were proposed for preparing selective modified electrodes [20][15]. MMIPs as selective adsorbents for SPE procedures offer advantages such as avoidance of drawbacks associated with conventional batch SPE/µ-SPE procedures, which need filtration/centrifugation steps for separating the adsorbent from the bulk sample after the loading stage and from the extract after analyte elution. In addition, losses of adsorbent particles are minimized since adsorbent separation is easily and quickly achieved by applying a magnet [21][16]. As previously mentioned, MMIP nanoparticles can be stirred (dispersed) in the sample/extract (loading step) and in the eluting solution (elution step), taking advantage of their magnetic properties, but stirring can be also performed by vortexing and by ultrasound dispersion. There are several strategies for preparing MMIPs, which lead to a great varietyof magnetic adsorbents. Moreover, despite free radical polymerization mechanism(s), which are mainly used to prepare MMIPs (and also MIPs), the heterogeneity caused by the fast chain propagation and irreversible termination reactions has led to the use of controlled radical polymerization strategies such as reversible addition fragmentation chain-transfer (RAFT) polymerization for preparing MIPs [22][17] and also MIP coatings over magnetic and non-magnetic supports [23,24,25,26][18][19][20][21]. RAFT polymerization provides more accessible sites for target adsorption and faster mass transfer because of the more homogenous polymeric network [27][22].2.1.1. Classification of MMIPs
Based on MMIP structure, four types of MMIPs can be established: core–shell MMIPs, magnetic nanotube-supported MIPs, magnetic nanosheet-supported MIPs, and magnetic hollow porous MIPs [28][23].
2.1.2. Magnetite Surface Functionalization for Core–Shell MMIPs
Magnetite surface functionalization can be performed mainly by using silica-based, diol-based, and vinyled compounds. However, there are other functionalization mechanisms as well as several combinations of surface modifier reagents for Fe3O4 nanoparticle surface functionalization.
Surface Functionalization with Hydroxyl (Diol) and Vinyl-Based Reagents
Diol-based reagents such as polyethylene glycol (PEG) [53,54,55,56,57][24][25][26][27][28] interact with the nanoparticle surface through one of the hydroxyl groups, allowing the remaining hydroxyl groups to be available to react with the components of the pre-polymerization mixture (Figure 2).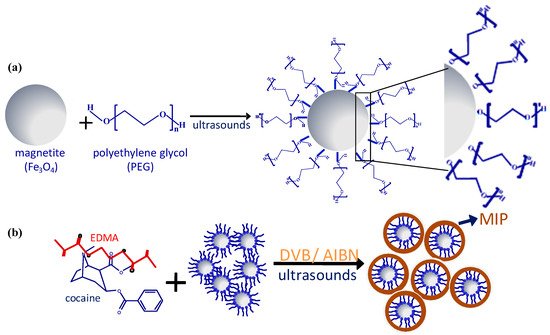
Surface Functionalization with Silica-Based Reagents
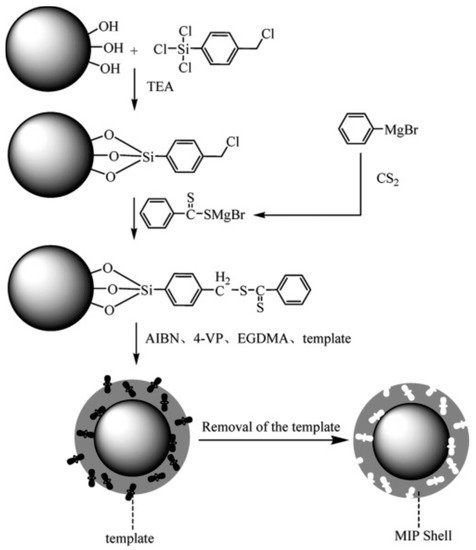
2.1.3. Magnetite Surface Functionalization for Magnetic Nanotube-Supported and Magnetic Nanosheet-Supported MIPs
| Fe3O4@OH Functionalization | |
|---|---|
| Diol-based reagents | Ref. |
| Polyethylene glycol (PEG) | [24][25][26][27][28][52] |
| Poly(vinyl alcohol) | [53] |
| Acrylic acid | [54] |
| Methacrylic acid (MAA) | [55] |
| Boronic acids: | |
| 2,4-Difluoro-3-formyl-phenylboronic acid (DFFPBA) a,b | [56][57] |
| 4-Formylphenylboronic acid (FPBA) plus sodium cyanoborohydride (NaBH3CN) | [58][59] |
| 4-Vinylphenboronic acid (VPBA) c | [60] |
| 3-Aminophenylboronic acid (APBA) d | [61] |
| Silica-based reagents | |
| Tetraethyl orthosilicate (TEOS) | [29][52][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][62] |
| Fe3O4@CH=C2H4 functionalization | |
| Oleic acid (OA) | [63][64][65][66][67][68][69][70][71][72][73][74] |
| Silica-based reagents: | |
| 3-(Trimethoxysilyl) propyl methacrylate (TMSMA) | [75] |
| 3-Methacryloxypropyltrimethoxysilane (MPS or KH-570) | [22][33][34][37][38][39][42][43][44][45][46][62][76][77][78][79][80][81][82][83][84][85][86] |
| Vinyl trimethoxy silane (VTMOS) | [34] |
| Vinyl triethoxy silane (VTEO or VTES) | [87][88][89][90] |
| Fe3O4@NH2 functionalization | |
| Silica-based reagents: | |
| (3-Aminopropyl)triethoxysilane (APTES) | [40][77][91][92][93][94][95][96] |
| Methacryloyl chloride | [97] |
| Fe3O4@COOH functionalization | |
| Silica-based reagents | |
| Poly(ethylene glycol)bis(carboxymethyl) ether e | [95] |
| Fe3O4@X, X= Cl or Br functionalization | |
| Silica-based reagents | |
| 4-Chloromethyl phenyl trichlorosilane (4-CPS) f | [36][39][98][99][100][101][102][103] |
| 3-Bromopropyl trimethoxy silane (BPTS) | [104] |
2.1.3. Magnetite Surface Functionalization for Magnetic Nanotube-Supported and Magnetic Nanosheet-Supported MIPs
Surface functionalization of mixed magnetic composites involving the presence of CNTs [31][105] and MWCNTs [143,144,145][106][107][108] has been efficiently achieved by using diol-based reagents such as EG and PEG [31[105][106][109][110],143,146,147], although some authors have described the convenience of a previous MWCNT@Fe3O4 composite oxidation [144][107], reduction [145][108], or carboxylation [32,148][111][112] stage before functionalization/MIP synthesis.
Regarding magnetic nanosheet-supported MIPs, the GO@Fe3O4 surface is usually functionalized by grafting with acrylic acid as shown in Figure 4 [33[113][114],151], which ensure the presence of vinyl groups for further polymerization. Acrylic acid was also used for surface modification of chitosan based GO@Fe3O4 composites [152][115].
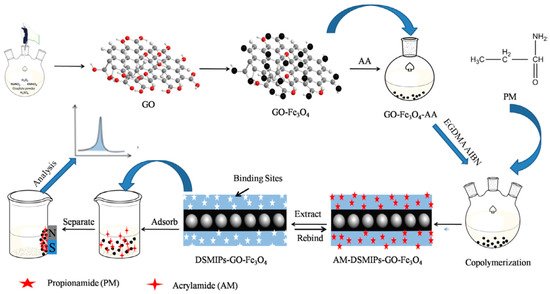
2.1.4. Magnetite Functionalization for Magnetic Porous MIPs
As previously commented, functionalization in HPMIPs based on mesoporous silica (1,2-diol groups over the HPMIPs) can be achieved by treating the composite with diluted perchloric acid (Figure 5) [37][116].
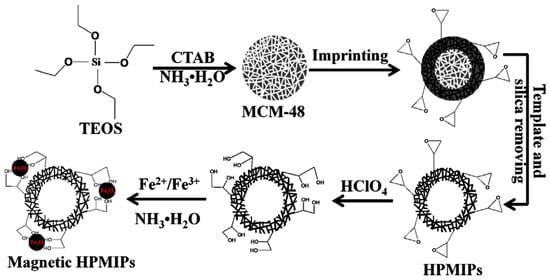
2.1.5. Other Mixed Composites for MMIPs
Various types of magnetic composites (Table 3) have been used as magnetic cores for MMIPs such as metal-organic frameworks (MOFs) and zeolite imidazolate frameworks (ZIFs).
2.2. Dispersive Solid Phase Extraction and Microsolid Phase Extraction with Non-Magnetic MIPs
dSPE/D-µ-SPE [9,10,11][3][4][5] can be performed by dispersing MMIP nanoparticles, and also non-magnetic MIP beads, by vortex and ultrasound stirring [10][4]. The adsorbents can be obtained by precipitation [166,167,168,169[117][118][119][120][121][122][123][124][125],170,171,172,173,174], and bulk [175,176,177][126][127][128] polymerization has been used for dSPE/D-µ-SPE by shaking the sample/extract-MIP bead mixtures for times varying from 5.0 min [166][117] to 3.0 h [169][120]. Absorption times can be reduced to 1 min when assisting the procedure by ultrasounds, enough time for isolating phenolic compounds in aqueous samples using 10 mg of MIP [170][121].
Ionic molecularly imprinted polymers (IIPs) have also been proposed for dSPE [173][124]. MIP synthesis around non-magnetic nanoparticles, such as silica nanoparticles, has been also performed to obtain stable adsorbents. In addition, the excellent properties of MOFs have led to preparation of MOF-MIP composites based on UiO-66 MOF [178][129] and HKUST-1 MOF [180][130] by direct MIP polymerization on the MOF’s surface. Hollow non-magnetic composites based on silica [180][130] and carbon [181][131] have been also prepared for dSPE/D-µ-SPE. Other composites such as MWCNT-MIPs have also been demonstrated to be effective adsorbents for dSPE of dioctyl phthalate in beverage samples [182][132].
3. Drawbacks and Future Prospects
MIMSPE procedures have been revealed as excellent approaches for miniaturization of SPE-based techniques in analytical chemistry, offering selective extraction/pre-concentration when analyzing complex samples. Dispersive SPE/µ-SPE procedures based on MIPs (mainly MMIPs) have shown high potential of miniaturization, which implies the use of low amounts of adsorbents as well as low volumes of organic solvents for performing the elution stage. However, MIPs and MMIPs face a number of challenges during the preparation (synthesis) stage and also during the application. MMIPs are synthesized in nonpolar solvents to avoid the disruption of the hydrogen bonding between monomer and templates. The generated hydrophobic surfaces lead to adsorption of interferences such as proteins. RAFT polymerization is a good alternative to overcome this problem since it allows the preparation of highly hydrophilic MIPs (or MIP external layers over nanoparticles), which can lead to efficient adsorbents for samples of a wide polarity range. Improvements have also been addressed to automate the techniques (similar to on-column/cartridges SPE) since batch MIMSPE procedures require several steps (conditioning, loading, washing, elution) and the procedures are not appealing processes when coping with hundreds of samples. In addition, the coupling (and also automation) of the MIMSPE devices directly with analytical instruments has not been explored yet. In any case, MIMSPE procedures open a fascinating window to analyzing compounds from complex matrices, and continuous efforts in this research area should open more and more novel applications.References
- Pawliszyn, J. New directions in sample preparation for analysis of organic compounds. Trends Anal. Chem. 1995, 14, 113–122.
- Turiel, E.; Martín-Esteban, A. Molecularly imprinted polymers-based microextraction techniques. Trends Anal. Chem. 2019, 118, 574–586.
- Ghorbani, M.; Aghamohammadhassan, M.; Chamsaz, M.; Akhlaghi, H.; Pedramrad, T. Dispersive solid phase microextraction. Trends Anal. Chem. 2019, 118, 793–809.
- Ghorbani, M.; Aghamohammadhassan, M.; Ghorbani, H.; Zabihi, A. Trends in sorbent development for dispersive micro-solid phase extraction. Microchem. J. 2020, 158, 105250.
- Ojeda, C.B.; Rojas, F.S. Vortex-Assisted Liquid–Liquid Microextraction (VALLME): The Latest Applications. Chromatographia 2017, 81, 89–103.
- Moreda-Piñeiro, J.; Moreda-Piñeiro, A. Combined assisted extraction techniques as green sample pre-treatments in food analysis. Trends Anal. Chem. 2019, 118, 1–18.
- Pichon, V.; Delaunay, N.; Combès, A. Sample Preparation Using Molecularly Imprinted Polymers. Anal. Chem. 2019, 92, 16–33.
- Adewuyi, Y.G. Sonochemistry: Environmental Science and Engineering Applications. Ind. Eng. Chem. Res. 2001, 40, 4681–4715.
- Galán-Cano, F.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Dispersive micro-solid phase extraction with ionic liquid-modified silica for the determination of organophosphate pesticides in water by ultra performance liquid chromatography. Microchem. J. 2013, 106, 311–317.
- Cai, Q.; Zhang, L.; Zhao, P.; Lun, X.; Li, W.; Guo, Y.; Hou, X. A joint experimental-computational investigation: Metal organic framework as a vortex assisted dispersive micro-solid-phase extraction sorbent coupled with UPLC-MS/MS for the simultaneous determination of amphenicols and their metabolite in aquaculture water. Microchem. J. 2017, 130, 263–270.
- Aghaie, A.B.; Hadjmohammadi, M.R. Fe3O4@p-Naphtholbenzein as a novel nano-sorbent for highly effective removal and recovery of Berberine: Response surface methodology for optimization of ultrasound assisted dispersive magnetic solid phase extraction. Talanta 2016, 156, 18–28.
- Dil, E.A.; Ghaedi, M.; Asfaram, A.; Mehrabi, F.; Bazrafshan, A.A.; Ghaedi, A.M. Trace determination of safranin O dye using ultrasound assisted dispersive solid-phase micro extraction: Artificial neural network-genetic algorithm and response surface methodology. Ultrason. Sonochem. 2016, 33, 129–140.
- Krawczyk, M.; Stanisz, E. Ultrasound-assisted dispersive micro solid-phase extraction with nano-TiO2 as adsorbent for the determination of mercury species. Talanta 2016, 161, 384–391.
- Ansell, R.J.; Mosbach, K. Magnetic molecularly imprinted polymer beads for drug radioligand binding assay. Analyst 1998, 123, 1611–1616.
- Zhang, L.; Li, J.; Zeng, Y. Molecularly imprinted magnetic nanoparticles for determination of the herbicide chlorotoluron by gate-controlled electro-catalytic oxidation of hydrazine. Microchim. Acta 2015, 182, 249–255.
- Martín-Esteban, A. Molecularly-imprinted polymers as a versatile, highly selective tool in sample preparation. Trends Anal. Chem. 2013, 45, 169–181.
- Yang, M.; Zhang, Y.; Lin, S.; Yang, X.; Fan, Z.; Yang, L.; Dong, X. Preparation of a bifunctional pyrazosulfuron-ethyl imprinted polymer with hydrophilic external layers by reversible addition–fragmentation chain transfer polymerization and its application in the sulfonylurea residue analysis. Talanta 2013, 114, 143–151.
- Lu, C.-H.; Zhou, W.-H.; Han, B.; Yang, H.-H.; Chen, X.; Wang, X.-R. Surface-Imprinted Core−Shell Nanoparticles for Sorbent Assays. Anal. Chem. 2007, 79, 5457–5461.
- Chang, L.; Li, Y.; Chu, J.; Qi, J.; Li, X. Preparation of core-shell molecularly imprinted polymer via the combination of reversible addition-fragmentation chain transfer polymerization and click reaction. Anal. Chim. Acta 2010, 680, 65–71.
- Zhang, H. Controlled/“living” radical precipitation polymerization: A versatile polymerization technique for advanced functional polymers. Eur. Polym. J. 2013, 49, 579–600.
- Abdollahi, E.; Abdouss, M.; Salami-Kalajahi, M.; Mohammadi, A. Molecular Recognition Ability of Molecularly Imprinted Polymer Nano- and Micro-Particles by Reversible Addition-Fragmentation Chain Transfer Polymerization. Polym. Rev. 2016, 56, 557–583.
- Azizi, A.; Shahhoseini, F.; Bottaro, C.S. Magnetic molecularly imprinted polymers prepared by reversible addition fragmentation chain transfer polymerization for dispersive solid phase extraction of polycyclic aromatic hydrocarbons in water. J. Chromatogr. A 2020, 1610, 460534.
- Huang, S.; Xu, J.; Zheng, J.; Zhu, F.; Xie, L.; Ouyang, G. Synthesis and application of magnetic molecularly imprinted polymers in sample preparation. Anal. Bioanal. Chem. 2018, 410, 3991–4014.
- Hu, Y.; Li, Y.; Liu, R.; Tan, W.; Li, G. Magnetic molecularly imprinted polymer beads prepared by microwave heating for selective enrichment of β-agonists in pork and pig liver samples. Talanta 2011, 84, 462–470.
- Wang, X.; Mao, H.; Huang, W.; Guan, W.; Zou, X.; Pan, J.; Yan, Y. Preparation of magnetic imprinted polymer particles via microwave heating initiated polymerization for selective enrichment of 2-amino-4-nitrophenol from aqueous solution. Chem. Eng. J. 2011, 178, 85–92.
- Sánchez-González, J.; Tabernero, M.J.; Bermejo, A.M.; Bermejo–Barrera, P.; Moreda–Piñeiro, A. Development of magnetic molecularly imprinted polymers for solid phase extraction of cocaine and metabolites in urine before high performance liquid chromatography—Tandem mass spectrometry. Talanta 2016, 147, 641–649.
- Sánchez-González, J.; Barreiro-Grille, T.; Cabarcos, P.; Tabernero-Duque, M.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Magnetic molecularly imprinted polymer based—Micro-solid phase extraction of cocaine and metabolites in plasma followed by high performance liquid chromatography—Tandem mass spectrometry. Microchem. J. 2016, 127, 206–212.
- Bagheri, A.R.; Arabi, M.; Ghaedi, M.; Ostovan, A.; Wang, X.; Li, J.; Chen, L. Dummy molecularly imprinted polymers based on a green synthesis strategy for magnetic solid-phase extraction of acrylamide in food samples. Talanta 2019, 195, 390–400.
- Niu, M.; Pham-Huy, C.; He, H. Core-shell nanoparticles coated with molecularly imprinted polymers: A review. Microchim. Acta 2016, 183, 2677–2695.
- Jing, T.; Du, H.; Dai, Q.; Xia, H.; Niu, J.; Hao, Q.; Mei, S.; Zhou, Y. Magnetic molecularly imprinted nanoparticles for recognition of lysozyme. Biosens. Bioelectron. 2010, 26, 301–306.
- Zhang, Z.; Luo, L.; Cai, R.; Chen, H. A sensitive and selective molecularly imprinted sensor combined with magnetic molecularly imprinted solid phase extraction for determination of dibutyl phthalate. Biosens. Bioelectron. 2013, 49, 367–373.
- Li, Y.; Dong, C.; Chu, J.; Qi, J.; Li, X. Surface molecular imprinting onto fluorescein-coated magnetic nanoparticles via reversible addition fragmentation chain transfer polymerization: A facile three-in-one system for recognition and separation of endocrine disrupting chemicals. Nanoscale 2011, 3, 280–287.
- Azodi-Deilami, S.; Abdouss, M.; Asadi, E.; Najafabadi, A.H.; Sadeghi, S.; Farzaneh, S.; Asadi, S. Magnetic molecularly imprinted polymer nanoparticles coupled with high performance liquid chromatography for solid-phase extraction of carvedilol in serum samples. J. Appl. Polym. Sci. 2014, 131.
- Azodi-Deilami, S.; Najafabadi, A.H.; Asadi, E.; Abdouss, M.; Kordestani, D. Magnetic molecularly imprinted polymer nanoparticles for the solid-phase extraction of paracetamol from plasma samples, followed its determination by HPLC. Microchim. Acta 2014, 181, 1823–1832.
- Fan, J.-P.; Xu, X.-K.; Xu, R.; Zhang, X.-H.; Zhu, J.-H. Preparation and characterization of molecular imprinted polymer functionalized with core/shell magnetic particles (Fe3O4@SiO2@MIP) for the simultaneous recognition and enrichment of four taxoids in Taxus × media. Chem. Eng. J. 2015, 279, 567–577.
- Xie, X.; Chen, L.; Pan, X.; Wang, S. Synthesis of magnetic molecularly imprinted polymers by reversible addition fragmentation chain transfer strategy and its application in the Sudan dyes residue analysis. J. Chromatogr. A 2015, 1405, 32–39.
- Miao, S.S.; Wu, M.S.; Zuo, H.G.; Jiang, C.; Jin, S.F.; Lu, Y.C.; Yang, H. Core–Shell Magnetic Molecularly Imprinted Polymers as Sorbent for Sulfonylurea Herbicide Residues. J. Agric. Food Chem. 2015, 63, 3634–3645.
- Uzuriaga-Sánchez, R.J.; Khan, S.; Wong, A.; Picasso, G.; Pividori, M.I.; Sotomayor, M.D.P.T. Magnetically separable polymer (Mag-MIP) for selective analysis of biotin in food samples. Food Chem. 2016, 190, 460–467.
- Yuan, Y.; Liu, Y.; Teng, W.; Tan, J.; Liang, Y.; Tang, Y. Preparation of core-shell magnetic molecular imprinted polymer with binary monomer for the fast and selective extraction of bisphenol A from milk. J. Chromatogr. A 2016, 1462, 2–7.
- Alcudia-León, M.D.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M.; Aranzana, M.S.C. Selective extraction of Bactrocera oleae sexual pheromone from olive oil by dispersive magnetic microsolid phase extraction using a molecularly imprinted nanocomposite. J. Chromatogr. A 2016, 1455, 57–64.
- Karimi, M.A.; Ranjbar, M.; Akbarpoor, M. Preparation of Magnetic Molecularly Imprinted Polymer Nanoparticles for Selective Adsorption and Separation of β-Estradiol. J. Clust. Sci. 2016, 27, 1067–1080.
- Haeri, S.A.; Abbasi, S. Biocoacervation extraction combined with dispersive solid phase extraction using a reversed-phase core-shell magnetic molecularly imprinted sorbent for 2,4-dichlorophenoxyacetic acid prior to its determination by HPLC. J. Iran. Chem. Soc. 2016, 13, 1993–1999.
- Tan, L.; He, R.; Chen, K.; Peng, R.; Huang, C.; Yang, R.; Tang, Y. Ultra-high performance liquid chromatography combined with mass spectrometry for determination of aflatoxins using dummy molecularly imprinted polymers deposited on silica-coated magnetic nanoparticles. Microchim. Acta 2016, 183, 1469–1477.
- Bazmandegan-Shamili, A.; Dadfarnia, S.; Shabani, A.M.H.; Saeidi, M.; Moghadam, M.R. High-Performance Liquid Chromatographic Determination of Diazinon after Its Magnetic Dispersive Solid-Phase Microextraction Using Magnetic Molecularly Imprinted Polymer. Food Anal. Methods 2016, 9, 2621–2630.
- Ben Aissa, A.; Herrera-Chacon, A.; Pupin, R.; Sotomayor, M.; Pividori, M. Magnetic molecularly imprinted polymer for the isolation and detection of biotin and biotinylated biomolecules. Biosens. Bioelectron. 2017, 88, 101–108.
- Wu, X.; Li, Y.; Zhu, X.; He, C.; Wang, Q.; Liu, S. Dummy molecularly imprinted magnetic nanoparticles for dispersive solid-phase extraction and determination of bisphenol A in water samples and orange juice. Talanta 2017, 162, 57–64.
- Men, H.-F.; Liu, H.-Q.; Zhang, Z.-L.; Huang, J.; Zhang, J.; Zhai, Y.-Y.; Li, L. Synthesis, properties and application research of atrazine Fe3O4@SiO2 magnetic molecularly imprinted polymer. Environ. Sci. Pollut. Res. 2012, 19, 2271–2280.
- Lu, C.; Tang, Z.; Gao, X.; Ma, X.; Liu, C. Computer-aided design of magnetic dummy molecularly imprinted polymers for solid-phase extraction of ten phthalates from food prior to their determination by GC-MS/MS. Microchim. Acta 2018, 185, 373.
- Luo, X.; Huang, Y.; Deng, F.; Luo, S.; Zhan, Y.; Shu, H.; Tu, X. A magnetic copper(II)-imprinted polymer for the selective enrichment of trace copper(II) ions in environmental water. Microchim. Acta 2012, 179, 283–289.
- Zhang, Y.-Z.; Zhang, J.; Tan, L.; Xia, Z.; Wang, C.-Z.; Zhou, L.-D.; Zhang, Q.; Yuan, C.-S. Preparation and evaluation of temperature and magnetic dual-responsive molecularly imprinted polymers for the specific enrichment of formononetin. J. Sep. Sci. 2018, 41, 3060–3068.
- Dil, E.A.; Doustimotlagh, A.H.; Javadian, H.; Asfaram, A.; Ghaedi, M. Nano-sized Fe3O4@SiO2-molecular imprinted polymer as a sorbent for dispersive solid-phase microextraction of melatonin in the methanolic extract of Portulaca oleracea, biological, and water samples. Talanta 2021, 221, 121620.
- Wu, X.; Wang, X.; Lu, W.; Wang, X.; Li, J.; You, H.; Xiong, H.; Chen, L. Water-compatible temperature and magnetic dual-responsive molecularly imprinted polymers for recognition and extraction of bisphenol A. J. Chromatogr. A 2016, 1435, 30–38.
- Zhang, Y.; Liu, R.; Hu, Y.; Li, G. Microwave Heating in Preparation of Magnetic Molecularly Imprinted Polymer Beads for Trace Triazines Analysis in Complicated Samples. Anal. Chem. 2009, 81, 967–976.
- Zhang, Y.; Cao, H.; Huang, Q.; Liu, X.; Zhang, H. Isolation of transferrin by imprinted nanoparticles with magnetic deep eutectic solvents as monomer. Anal. Bioanal. Chem. 2018, 410, 6237–6245.
- Safdarian, M.; Ramezani, Z. Rapid microwave-assisted distillation–precipitation polymerization for the synthesis of magnetic molecular imprinted polymers coupled to HPTLC determination of perphenazine in human urine. New J. Chem. 2018, 43, 48–57.
- Li, D.; Yuan, Q.; Yang, W.; Yang, M.; Li, S.; Tu, T. Efficient vitamin B12-imprinted boronate affinity magnetic nanoparticles for the specific capture of vitamin B12. Anal. Biochem. 2018, 561, 18–26.
- Bie, Z.; Xing, R.; He, X.; Ma, Y.; Chen, Y.; Liu, Z. Precision Imprinting of Glycopeptides for Facile Preparation of Glycan-Specific Artificial Antibodies. Anal. Chem. 2018, 90, 9845–9852.
- Hu, J.; Zhu, S.; Chen, S.-E.; Liu, R.; Sun, J.; Zhao, X.-E.; Liu, H. Multiplexed derivatization strategy-based dummy molecularly imprinted polymers as sorbents for magnetic dispersive solid phase extraction of globotriaosylsphingosine prior to UHPLC-MS/MS quantitation. Microchim. Acta 2020, 187, 373.
- Sun, X.-Y.; Ma, R.-T.; Chen, J.; Shi, Y.-P. Boronate-affinity based magnetic molecularly imprinted nanoparticles for the efficient extraction of the model glycoprotein horseradish peroxidase. Microchim. Acta 2017, 184, 3729–3737.
- Sun, X.-Y.; Ma, R.-T.; Chen, J.; Shi, Y.-P. Magnetic boronate modified molecularly imprinted polymers on magnetite microspheres modified with porous TiO2 (Fe3O4@pTiO2@MIP) with enhanced adsorption capacity for glycoproteins and with wide operational pH range. Microchim. Acta 2018, 185, 565.
- Huang, W.; Hou, X.; Tong, Y.; Tian, M. Determination of sialic acid in serum samples by dispersive solid-phase extraction based on boronate-affinity magnetic hollow molecularly imprinted polymer sorbent. RSC Adv. 2019, 9, 5394–5401.
- Attallah, O.A.; Al-Ghobashy, M.A.; Ayoub, A.T.; Nebsen, M. Magnetic molecularly imprinted polymer nanoparticles for simultaneous extraction and determination of 6-mercaptopurine and its active metabolite thioguanine in human plasma. J. Chromatogr. A 2018, 1561, 28–38.
- Medina-Castillo, A.L.; Mistlberger, G.; Fernandez-Sanchez, J.F.; Carretero, A.S.; Klimant, I.; Gutierrez, A.F. Novel Strategy To Design Magnetic, Molecular Imprinted Polymers with Well-Controlled Structure for the Application in Optical Sensors. Macromolecules 2010, 43, 55–61.
- Chen, L.; Zhang, X.; Xu, Y.; Du, X.; Sun, X.; Sun, L.; Wang, H.; Zhao, Q.; Yu, A.; Zhang, H.; et al. Determination of fluoroquinolone antibiotics in environmental water samples based on magnetic molecularly imprinted polymer extraction followed by liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2010, 662, 31–38.
- Zhang, X.; Chen, L.; Xu, Y.; Wang, H.; Zeng, Q.; Zhao, Q.; Ren, N.; Ding, L. Determination of β-lactam antibiotics in milk based on magnetic molecularly imprinted polymer extraction coupled with liquid chromatography—Tandem mass spectrometry. J. Chromatogr. B 2010, 878, 3421–3426.
- Gu, X.-H.; Xu, R.; Yuan, G.-L.; Lu, H.; Gu, B.-R.; Xie, H.-P. Preparation of chlorogenic acid surface-imprinted magnetic nanoparticles and their usage in separation of Traditional Chinese Medicine. Anal. Chim. Acta 2010, 675, 64–70.
- Pan, J.; Xu, L.; Dai, J.; Li, X.; Hang, H.; Huo, P.; Li, C.; Yan, Y. Magnetic molecularly imprinted polymers based on attapulgite/Fe3O4 particles for the selective recognition of 2,4-dichlorophenol. Chem. Eng. J. 2011, 174, 68–75.
- Liu, J.; Wang, W.; Xie, Y.; Huang, Y.; Liu, Y.; Liu, X.; Zhao, R.; Liu, G.; Chen, Y. A novel polychloromethylstyrene coated superparamagnetic surface molecularly imprinted core–shell nanoparticle for bisphenol A. J. Mater. Chem. 2011, 21, 9232–9238.
- Cheng, X.; Yan, H.; Wang, X.; Sun, N.; Qiao, X. Vortex-assisted magnetic dispersive solid-phase microextraction for rapid screening and recognition of dicofol residues in tea products. Food Chem. 2014, 162, 104–109.
- Lahcen, A.A.; Baleg, A.A.; Baker, P.; Iwuoha, E.; Amine, A. Synthesis and electrochemical characterization of nanostructured magnetic molecularly imprinted polymers for 17-β-Estradiol determination. Sens. Actuators B Chem. 2017, 241, 698–705.
- Lee, M.-H.; Thomas, J.L.; Ho, M.-H.; Yuan, C.; Lin, H.-Y. Synthesis of Magnetic Molecularly Imprinted Poly(ethylene-co-vinyl alcohol) Nanoparticles and Their Uses in the Extraction and Sensing of Target Molecules in Urine. ACS Appl. Mater. Interfaces 2010, 2, 1729–1736.
- Uzuriaga-Sánchez, R.J.; Wong, A.; Khan, S.; Pividori, M.I.; Picasso, G.; Sotomayor, M.D. Synthesis of a new magnetic-MIP for the selective detection of 1-chloro-2,4-dinitrobenzene, a highly allergenic compound. Mater. Sci. Eng. C 2017, 74, 365–373.
- Peyrovi, M.; Hadjmohammadi, M.; Saeidi, I. Synthesis of magnetic nanoparticle-based molecularly imprinted polymer as a selective sorbent for efficient extraction of ezetimibe from biological samples. Biomed. Chromatogr. 2019, 33, e4404.
- Ilktaç, R.; Gumus, Z.P.; Aksuner, N.; Coskunol, H. Highly sensitive and selective method for the rapid determination and preconcentration of haloperidol by using a magnetite-molecularly imprinted polymer. J. Sep. Sci. 2019, 42, 2115–2122.
- Luo, X.; Deng, F.; Luo, S.; Tu, X.; Yang, L. Grafting of molecularly imprinted polymers from the surface of Fe3O4 nanoparticles containing double bond via suspension polymerization in aqueous environment: A selective sorbent for theophylline. J. Appl. Polym. Sci. 2011, 121, 1930–1937.
- Zhang, R.; Zhang, T.; Lv, Y.; Qin, P.; Li, H.; Li, J.-P.; Tan, T. Selective binding of heparin oligosaccharides in a magnetic thermoresponsive molecularly imprinted polymer. Talanta 2019, 201, 441–449.
- He, Y.; Zhao, F.; Zhang, C.; Abd EI-Aty, A.M.; Baranenko, D.A.; Hacimüftüoğlu, A.; She, Y. Assessment of magnetic core-shell mesoporous molecularly imprinted polymers for selective recognition of triazoles residual levels in cucumber. J. Chromatogr. B 2019, 1132, 121811.
- Garcia, R.; Carreiro, E.P.; Ramalho, J.P.P.; Mirao, J.; Burke, A.; da Silva, M.D.R.G.; Freitas, A.M.C.; Cabrita, M.J. A magnetic controllable tool for the selective enrichment of dimethoate from olive oil samples: A responsive molecular imprinting-based approach. Food Chem. 2018, 254, 309–316.
- Zhao, Q.-Y.; Zhao, H.-T.; Yang, X.; Zhang, H.; Dong, A.-J.; Wang, J.; Li, B. Selective recognition and fast enrichment of anthocyanins by dummy molecularly imprinted magnetic nanoparticles. J. Chromatogr. A 2018, 1572, 9–19.
- Li, Z.; Lei, C.; Wang, N.; Jiang, X.; Zeng, Y.; Fu, Z.; Zou, L.; He, L.; Liu, S.; Ao, X.; et al. Preparation of magnetic molecularly imprinted polymers with double functional monomers for the extraction and detection of chloramphenicol in food. J. Chromatogr. B 2018, 1100, 113–121.
- Wang, H.; Yuan, L.; Zhu, H.; Jin, R.; Xing, J. Comparative study of capsaicin molecularly imprinted polymers prepared by different polymerization methods. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 157–164.
- Zhong, M.; Wang, Y.-H.; Wang, L.; Long, R.-Q.; Chen, C.-L. Synthesis and characterization of magnetic molecularly imprinted polymers for enrichment of sanguinarine from the extraction wastewater of M. cordata. J. Ind. Eng. Chem. 2018, 66, 107–115.
- Yang, W.; Muhammad, T.; Yigaimu, A.; Muhammad, K.; Chen, L. Preparation of stoichiometric molecularly imprinted polymer coatings on magnetic particles for the selective extraction of auramine O from water. J. Sep. Sci. 2018, 41, 4185–4193.
- Xie, X.; Hu, Q.; Ke, R.; Zhen, X.; Bu, Y.; Wang, S. Facile preparation of photonic and magnetic dual responsive protein imprinted nanomaterial for specific recognition of bovine haemoglobin. Chem. Eng. J. 2019, 371, 130–137.
- Lu, Y.C.; Guo, M.H.; Mao, J.H.; Xiong, X.H.; Liu, Y.J.; Li, Y. Preparation of core-shell magnetic molecularly imprinted polymer nanoparticle for the rapid and selective enrichment of trace diuron from complicated matrices. Ecotoxicol. Environ. Saf. 2019, 177, 66–76.
- Qin, D.; Wang, J.; Ge, C.; Lian, Z. Fast extraction of chloramphenicol from marine sediments by using magnetic molecularly imprinted nanoparticles. Microchim. Acta 2019, 186, 428.
- Xu, W.; Wang, Y.; Wei, X.; Chen, J.; Xu, P.; Ni, R.; Meng, J.; Zhou, Y. Fabrication of magnetic polymers based on deep eutectic solvent for separation of bovine hemoglobin via molecular imprinting technology. Anal. Chim. Acta 2019, 1048, 1–11.
- Yu, X.; Liu, H.; Diao, J.; Sun, Y.; Wang, Y. Magnetic molecularly imprinted polymer nanoparticles for separating aromatic amines from azo dyes—Synthesis, characterization and application. Sep. Purif. Technol. 2018, 204, 213–219.
- Landarani, M.; Asgharinezhad, A.A.; Ebrahimzadeh, H. A magnetic ion-imprinted polymer composed of silica-coated magnetic nanoparticles and polymerized 4-vinyl pyridine and 2,6-diaminopyridine for selective extraction and determination of lead ions. New J. Chem. 2020, 44, 7561–7568.
- Bazmandegan-Shamili, A.; Dadfarnia, S.; Shabani, A.M.H.; Moghadam, M.R.; Saeidi, M. MultiSimplex optimization of the dispersive solid-phase microextraction and determination of fenitrothion by magnetic molecularly imprinted polymer and high-performance liquid chromatography. J. Iran. Chem. Soc. 2018, 15, 1181–1189.
- Hu, C.; Yang, Z.; Yan, F.; Sun, B. Extraction of the toluene exposure biomarkers hippuric acid and methylhippuric acid using a magnetic molecularly imprinted polymer, and their quantitation by LC-MS/MS. Microchim. Acta 2019, 186, 135.
- Mirzajani, R.; Keshavarz, A. The core–shell nanosized magnetic molecularly imprinted polymers for selective preconcentration and determination of ciprofloxacin in human fluid samples using a vortex-assisted dispersive micro-solid-phase extraction and high-performance liquid chromatography. J. Iran. Chem. Soc. 2019, 16, 2291–2306.
- Zhang, Z.; Wang, H.; Wang, H.; Wu, C.; Lia, M.; Li, L. Fabrication and evaluation of molecularly imprinted magnetic nanoparticles for selective recognition and magnetic separation of lysozyme in human urine. Analyst 2018, 143, 5849–5856.
- Asfaram, A.; Arabi, M.; Ostovan, A.; Sadeghi, H.; Ghaedi, M. Simple and selective detection of quercetin in extracts of plants and food samples by dispersive-micro-solid phase extraction based on core–shell magnetic molecularly imprinted polymers. New J. Chem. 2018, 42, 16144–16153.
- He, Y.; Tan, S.; Abd EI-Aty, A.M.; Hacımüftüoğlud, A.; She, Y. Magnetic molecularly imprinted polymers for the detection of aminopyralid in milk using dispersive solid-phase extraction. RSC Adv. 2019, 9, 29998.
- Chen, F.; Wang, J.; Lu, R.; Chen, H.; Xie, X. Fast and high-efficiency magnetic surface imprinting based on microwave-accelerated reversible addition fragmentation chain transfer polymerization for the selective extraction of estrogen residues in milk. J. Chromatogr. A 2018, 1562, 19–26.
- Cheng, Y.; Nie, J.; Li, J.; Liu, H.; Yan, Z.; Kuang, L. Synthesis and characterization of core–shell magnetic molecularly imprinted polymers for selective recognition and determination of quercetin in apple samples. Food Chem. 2019, 287, 100–106.
- Li, Y.; Li, X.; Chu, J.; Dong, C.; Qi, J.; Yuan, Y. Synthesis of core-shell magnetic molecular imprinted polymer by the surface RAFT polymerization for the fast and selective removal of endocrine disrupting chemicals from aqueous solutions. Environ. Pollut. 2010, 158, 2317–2323.
- Li, J.; Dong, R.; Wang, X.; Xiong, H.; Xu, S.; Shen, D.; Song, X.; Chen, L. One-pot synthesis of magnetic molecularly imprinted microspheres by RAFT precipitation polymerization for the fast and selective removal of 17b-estradiol. RSC Adv. 2015, 5, 10611.
- Chen, F.; Zhang, J.; Wang, M.; Kong, J. Magnetic molecularly imprinted polymers synthesized by surface-initiated reversible addition-fragmentation chain transfer polymerization for the enrichment and determination of synthetic estrogens in aqueous solution. J. Sep. Sci. 2015, 38, 2670–2676.
- Xie, X.; Liu, X.; Pan, X.; Chen, L.; Wang, S. Surface-imprinted magnetic particles for highly selective sulfonamides recognition prepared by reversible addition fragmentation chain transfer polymerization. Anal. Bioanal. Chem. 2015, 408, 963–970.
- Du, W.; Zhang, B.; Guo, P.; Chen, G.; Chang, C.; Fu, Q. Facile preparation of magnetic molecularly imprinted polymers for the selective extraction and determination of dexamethasone in skincare cosmetics using HPLC. J. Sep. Sci. 2018, 41, 2441–2452.
- Xiong, H.; Guo, L.; Mao, X.; Tan, T.; Wan, H.; Wan, Y. A magnetic hydrophilic molecularly imprinted material with multiple stimuli-response properties for efficient recognition of bisphenol A in beverages. Food Chem. 2020, 331, 127311.
- Turan, E.; Şahin, F. Molecularly imprinted biocompatible magnetic nanoparticles for specific recognition of Ochratoxin A. Sensors Actuators B Chem. 2016, 227, 668–676.
- Ma, G.; Chen, L. Development of magnetic molecularly imprinted polymers based on carbon nanotubes—Application for trace analysis of pyrethroids in fruit matrices. J. Chromatogr. A 2014, 1329, 1–9.
- Rao, W.; Cai, R.; Yin, Y.; Long, F.; Zhang, Z. Magnetic dummy molecularly imprinted polymers based on multi-walled carbon nanotubes for rapid selective solid-phase extraction of 4-nonylphenol in aqueous samples. Talanta 2014, 128, 170–176.
- Bahrani, S.; Ghaedi, M.; Mansoorkhani, M.J.K.; Ostovan, A. A highly selective nanocomposite based on MIP for curcumin trace levels quantification in food samples and human plasma following optimization by central composite design. J. Chromatogr. B 2017, 1040, 129–135.
- Kolaei, M.; Dashtian, K.; Rafiee, Z.; Ghaedi, M. Ultrasonic-assisted magnetic solid phase extraction of morphine in urine samples by new imprinted polymer-supported on MWCNT-Fe3O4-NPs: Central composite design optimization. Ultrason. Sonochem. 2016, 33, 240–248.
- Xiao, D.; Wang, C.; Dai, H.; Peng, J.; He, J.; Zhang, K.; Kong, S.; Qiu, P.; He, H. Applications of magnetic surface imprinted materials for solid phase extraction of levofloxacin in serum samples. J. Mol. Recognit. 2015, 28, 277–284.
- Xiao, D.; Dramou, P.; Xiong, N.; He, H.; Li, H.; Yuan, D.; Dai, H. Development of novel molecularly imprinted magnetic solid-phase extraction materials based on magnetic carbon nanotubes and their application for the determination of gatifloxacin in serum samples coupled with high performance liquid chromatography. J. Chromatogr. A 2013, 1274, 44–53.
- Ansari, S.; Masoum, S. A multi-walled carbon nanotube-based magnetic molecularly imprinted polymer as a highly selective sorbent for ultrasonic-assisted dispersive solid-phase microextraction of sotalol in biological fluids. Analyst 2018, 143, 2862–2875.
- Sedghi, R.; Heidari, B.; Kazemi, S. Novel magnetic ion-imprinted polymer: An efficient polymeric nanocomposite for selective separation and determination of Pb ions in aqueous media. Environ. Sci. Pollut. Res. 2018, 25, 26297–26306.
- Ning, F.; Qiu, T.; Wang, Q.; Peng, H.; Li, Y.; Wu, X.; Zhang, Z.; Chen, L.; Xiong, H. Dummy-surface molecularly imprinted polymers on magnetic graphene oxide for rapid and selective quantification of acrylamide in heat-processed (including fried) foods. Food Chem. 2017, 221, 1797–1804.
- Fan, J.-P.; Liao, D.-D.; Xie, Y.-L.; Zheng, B.; Yu, J.-X.; Cao, Y.-H.; Zhang, X.-H.; Peng, H.-L. A molecular imprinted polymer on the surface of superparamagnetic Fe3O4-graphene oxide ([email protected]3O4@GO) for simultaneous recognition and enrichment of evodiamine and rutaecarpine inEvodiae fructus. J. Appl. Polym. Sci. 2016, 134.
- Barati, A.; Kazemi, E.; Dadfarnia, S.; Shabani, A.M.H. Synthesis/characterization of molecular imprinted polymer based on magnetic chitosan/graphene oxide for selective separation/preconcentration of fluoxetine from environmental and biological samples. J. Ind. Eng. Chem. 2017, 46, 212–221.
- Li, H.; Hu, X.; Zhang, Y.; Shi, S.; Jiang, X.; Chen, X. High-capacity magnetic hollow porous molecularly imprinted polymers for specific extraction of protocatechuic acid. J. Chromatogr. A 2015, 1404, 21–27.
- Chen, C.; Zhang, X.; Long, Z.; Zhang, J.; Zheng, C. Molecularly imprinted dispersive solid-phase microextraction for determination of sulfamethazine by capillary electrophoresis. Microchim. Acta 2012, 178, 293–299.
- Song, X.; Zhou, T.; Li, J.; Su, Y.; Xie, J.; He, L. Determination of macrolide antibiotics residues in pork using molecularly imprinted dispersive solid-phase extraction coupled with LC-MS/MS. J. Sep. Sci. 2018, 41, 1138–1148.
- Liu, X.; Wang, Y.; Wang, J.; Li, L.; Li, R. Hydrophilic molecularly imprinted dispersive solid-phase extraction coupled with liquid chromatography for determination of azoxystrobin residues in cucumber. Iran. Polym. J. 2019, 28, 725–734.
- Wang, D.; Luo, X.; Wang, M.; Zhou, K.; Xia, Z. Selective separation and purification of polydatin by molecularly imprinted polymers from the extract of Polygoni Cuspidati Rhizoma et Radix, rats’ plasma and urine. J. Chromatogr. B 2020, 1156, 122307.
- Lu, W.; Ming, W.; Zhang, X.; Chen, L. Molecularly imprinted polymers for dispersive solid-phase extraction of phenolic compounds in aqueous samples coupled with capillary electrophoresis. Electrophoresis 2016, 37, 2487–2495.
- Jayasinghe, G.D.T.M.; Domínguez-González, R.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Miniaturized vortex assisted-dispersive molecularly imprinted polymer micro-solid phase extraction and HPLC-MS/MS for assessing trace aflatoxins in cultured fish. Anal. Methods 2020, 12, 4351.
- Lu, W.; Liu, J.; Li, J.; Wang, X.; Lv, M.; Cui, R.; Chen, L. Dual-template molecularly imprinted polymers for dispersive solid-phase extraction of fluoroquinolones in water samples coupled with high performance liquid chromatography. Analyst 2019, 144, 1292–1302.
- Jinadasa, K.K.; Peña-Vázquez, E.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Ionic imprinted polymer—Vortex-assisted dispersive micro-solid phase extraction for inorganic arsenic speciation in rice by HPLC-ICP-MS. Talanta 2020, 220, 121418.
- Wang, J.; Wang, Y.; Liu, X.-X.; Li, D.-M.; Li, S.-X. Molecularly imprinted dispersive solid-phase extraction coupled with high-performance liquid chromatography for the determination of pyraclostrobin in ginseng. Chem. Pap. 2020, 74, 1717–1727.
- Nezhadali, A.; Es’Haghi, Z.; Khatibi, A. Selective extraction of progesterone hormones from environmental and biological samples using a polypyrrole molecularly imprinted polymer and determination by gas chromatography. Anal. Methods 2016, 8, 1813–1827.
- Panjan, P.; Monasterio, R.P.; Carrasco-Pancorbo, A.; Fernandez-Gutierrez, A.; Sesay, A.M.; Fernandez-Sanchez, J.F. Development of a folic acid molecularly imprinted polymer and its evaluation as a sorbent for dispersive solid-phase extraction by liquid chromatography coupled to mass spectrometry. J. Chromatogr. A 2018, 1576, 26–33.
- Yu, X.-R.; Zhang, Z.-M.; Li, W.; Zhang, R.-R.; Jiao, H.-F.; Zhao, J.; Sun, A.-L.; Shi, X.-Z.; Chen, J. Development and Application of the dispersive solid-phase extraction method based on molecular imprinted polymers for removal of matrix components of bivalve shellfish extracts in the GC–MS/MS analysis of amide/dinitroaniline/substituted urea herbicides. Chromatographia 2019, 82, 961–970.
- Ma, N.; Feng, C.; Qu, P.; Wang, G.; Liu, J.; Liu, J.X.; Wang, J.P. Determination of Tetracyclines in Chicken by Dispersive Solid Phase Microextraction Based on Metal-Organic Frameworks/Molecularly Imprinted Nano-polymer and Ultra Performance Liquid Chromatography. Food Anal. Methods 2020, 13, 1211–1219.
- Chen, W.; Xue, M.; Xue, F.; Mu, X.; Xu, Z.; Meng, Z.; Zhu, G.; Shea, K.J. Molecularly imprinted hollow spheres for the solid phase extraction of estrogens. Talanta 2015, 140, 68–72.
- Gholami, H.; Ghaedi, M.; Ostovan, A.; Arabi, M.; Bagheri, A.R. Preparation of hollow porous molecularly imprinted and aluminum(III) doped silica nanospheres for extraction of the drugs valsartan and losartan prior to their quantitation by HPLC. Microchim. Acta 2019, 186, 702.
- Du, J.; Gao, R.; Mu, H. A Novel Molecularly Imprinted Polymer Based on Carbon Nanotubes for Selective Determination of Dioctyl Phthalate from Beverage Samples Coupled with GC/MS. Food Anal. Methods 2016, 9, 2026–2035.
