In the last decade, nanotechnological approaches have emerged as promising tools to tackle new challenges in cancer research, most prominently the efficient translation of the more in-depth fundamental understanding of cancer biology into practical clinical applications
[4][5][4,5]. Within the field of nanoscience, surface-enhanced Raman scattering (SERS) spectroscopy has gained prominence in biosensing
[6][7][8][9][6,7,8,9]. In SERS, the excitation of localized surface plasmon resonances (LSPR) at the surface of nanostructured metals with light induces the massive intensification of the Raman scattering from molecules located in close proximity to the metallic surface. This effect yields an ultrasensitive plasmon-enhanced spectroscopic technique which retains the intrinsic structural specificity and experimental flexibility of Raman spectroscopy
[10]. As impressive advances in instrumentation and nanofabrication techniques enabling the engineering of finely-tuned plasmonic nanomaterials continue, SERS is progressively expanding into the realm of viable biomedical applications. In cancer research, SERS is typically implemented in place of, or in combination with, fluorescence spectroscopy to address key limitations of such established technique. Most prominently, SERS sensing entails larger multiplexing capabilities for simultaneous quantitative interrogation at high imaging resolution (using a single excitation wavelength) as well as improved photostability, sensitivity and reduced tissue damages when employing near-infrared (NIR) lasers. On the other hand, it is worth highlighting that fluorescence imaging is intrinsically faster while currently maintaining a much larger availability of commercial products and established analytical protocols.
2. SERS Imaging of Cancer Tissues and Single Cells
The most straightforward and widely investigated implementation of SERS to cancer-related applications is as an optical imaging technique for molecular characterization of tumor tissues in place of (or in combination with) fluorescence spectroscopy. SERS-encoded particles (SEPs) may substitute for fluorescent reporters
[11][12][13][11,12,13] (typically, fluorescently-labelled antibodies selective for specific protein receptors on the cell surface) as contrast agents. SEPs always combine a SERS molecular code, yielding a unique vibrational fingerprint (signal read-out), bound to a plasmonic core (mostly, silver or gold), as required for the enhancement of the weak Raman signal (
Figure 1A). Additionally, an external protective layer (mainly, silica or polymeric) can be engineered around the encoded particles to improve stability while providing an optimal anchoring surface for further functionalization
[14][15][16][14,15,16]. In fact, surface elements are typically further integrated on such outer shell to generate convenient physicochemical properties, such as selective targeting via functional molecules (e.g., antibodies, aptamers, folates etc.)
[9][16][17][9,16,17].
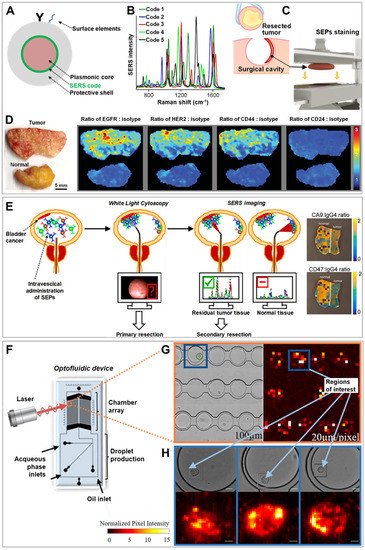
Figure 1. (
A) Schematic depiction of the traditional surface-enhanced Raman scattering (SERS)-encoded nanoparticle (SEP) construction. (
B–
D) Application of SERS imaging to resected tumor tissue for intraoperative surgical guidance: (
B) Unique SERS spectra of five different codes on gold particles coated with a silica shell. (
C) Upon excision, the resected sample is placed in an automated staining device combining multiple quick dipping of the specimen into a SEPs solution with high-frequency vibration for fast and extensive topically applications of encoded particles onto the surface of the fresh tissue. (
D) Ratiometric images biomarker targeting SEPs vs. negative control (isotype) obtained from raster-scanned SERS imaging (<3 min) of the illustrated human breast tumor and normal tissue. Adapted with permission from
[18]. Copyright 2016, Wiley-VHC. (
E) Schematic of the proposed application of intravesical SERS imaging for intraoperative endoscopic surgery. Adapted with permission from
[19]. Copyright 2018, American Chemical Society. (
F–
H) SERS-microfluidic device for single live cell analysis: (
F) Schematic depiction of the droplet-based optofluidic device; (
G) Low-resolution map of the chamber array, (
H) High-resolution map of individual cells encapsulated in droplets. Adapted with permission from
[20] Copyright 2018, American Chemical Society.
The optical efficiency of SEPs is strictly related to the geometric features of the plasmonic core (particle compositional nature, size and shape, as well as interparticle interactions)
[21][22][21,22]. Broadly speaking, the upper limit to the signal enhancement boosts as the nanoparticle is reshaped from spherical to anisotropic shapes (e.g., nanorods or nanostars) and, beyond, to nanoparticle aggregates. Concurrently, however, increasingly complicated practical challenges are posed for generating a broad panel of SEPs with reproducible and homogenous responses at a sufficient scale. In general, reliable and reproducible fabrication of nanomaterials in large quantities via standardized procedures still represents a bottleneck for implementation of nanomedicine. This explains why individual spherical nanoparticle cores remain the main preferred choice as optical enhancers in SEP-based applications (
Table S1 provides a list of diverse plasmonic core geometries from SEPs employed in cancer-related research). We refer the interested readers to excellent reviews
[14][15][16][17][14,15,16,17] for a more in-depth discussion on SEP fabrication, properties and applications.
The toxicity of nanomaterials is directly correlated with their retention and clearance. In the case of noble metal nanoparticles, a rapid and efficient excretion from the body is observed by reducing the nanoparticle size below the threshold for renal clearance (i.e., ca. < 5 nm)
[23]. Interestingly, biodegradable assemblies of ultrasmall gold nanoparticles have shown the possibility of tuning the plasmonic response of the nanomaterial in the NIR region while avoiding long-time persistence in the body
[24]. However, larger particle sizes are required to sustain LSPRs affording adequate electromagnetic enhancements. Thus, SEPs are characterized by relatively large intrinsic hydrodynamic diameters (typically > 100 nm) which currently prevents the clinical translation of SERS imaging of tumor tissues exploiting systemic routes of administration (e.g., intravenous injection) due to the major accumulation of metal particles in the body. Thus, major efforts have been recently focused on applications where SEPs can be directly delivered onto accessible tissues (e.g., via topical administration or direct intratumoral injection). Such approach drastically reduces the amount of nanomaterials required for the analysis thereby making the systemic uptake of the particles negligible
[9][25][26][27][28][9,25,26,27,28].
In this regard, a remarkable example has been recently reported by Wang et al.
[18] who developed a topical-staining protocol for rapid and quantitative multiplex SERS imaging of cancer biomarkers at the surfaces of freshly resected tissues to be used as an intraoperative tool for surgical guidance. Complete removal of a tumor at the primary site is a major issue in surgical oncology, as residual malignant microscopic foci may increase both the risk of recurrence and the rate of re-excision surgeries
[29]. As a result, tumor removal is typically performed resecting also an area of surrounding normal tissue which, however, may compromise patient health and cosmesis. Current intraoperative pathology examination of frozen-sections fails to provide the required level of specificity, reliability and speediness, and a complete assessment for the presence of residual tumor can only be performed postoperatively. To tackle this problem, the authors prepared five differently SEPs, all consisting of a 60 nm spherical gold core, functionalized with a specific SERS code yielding a unique vibrational fingerprint (
Figure 1B), and coated with a 30 nm silica layer. Monoclonal antibodies recognizing surface biomarkers overexpressed by breast cancer cells (EGFR, HER2, CD44 and CD24) were separately conjugated on four different SEPs. The last batch of contrast agents was functionalized with an antibody that does not actively target human proteins (IgG1, isotype control). Fast (<5 min) and extensive binding of SEPs at the surface of fresh human breast cancer tissues was achieved by repeatedly dipping the tissue into a solution of all contrast agents followed by high-frequency mechanical vibration (
Figure 1C). Specimen were then rapidly imaged yielding, upon spectral deconvolution, ratiometric SERS maps depicting the intensity ratio of each of the four actively targeting SEPs against the background of non-targeted particles (
Figure 1D). These maps precisely quantify the biomarker distributions, in agreement with immunohistochemistry data. Importantly, the use of the ratiometric approach, rather than the simple imaging of the absolute SERS signals, is required to address uneven and non-specific particle binding, removing then the need for extensive washing procedures. Following the same rationale, in vivo SERS imaging has been successfully applied in mice bearing ovarian adenocarcinoma on the peritoneum by Oseledchyk et al.
[28]. A mixture of one tumor targeting and one non-targeting SEPs were directly injected in the peritoneal cavity, enabling the acquisition of radiometric images that accurately identify the location of tumor lesions in the tissue.
A particularly promising exploitation of SERS imaging is via Raman endoscopy for minimally invasive real-time molecular characterization of the surface of hollow organs. Currently, the “gold standard” for endoscopic procedures (white-light endoscopy, WLE) suffers from major limitations at detecting small tumor lesions, therefore requiring postoperative histological examinations to establish clinical decisions
[30]. Notably, conventional clinical endoscopes can be suitably integrated with optical fibers for remote optical imaging and additional channels for local particle administration
[27][31][27,31]. As a recent example, the feasibility of this approach has been demonstrated for multiplex imaging of bladder cancer tissue during transurethral resection
[19]. Gambhir and co-workers administered ex-vivo in human bladders a cocktail of three antibody-conjugated SEPs (two actively targeting surface protein biomarkers CA9 and CD47, and one as control), acquiring ratiometric SERS via a Raman endoscope that yield accurate classification of tumor vs normal tissue. The proposed clinical application is outlined in
Figure 1E. Upon pre-administration of SEPs into the patient bladder, transurethral cancer removal is carried out assisted by WLE. Subsequently, SERS mapping is performed using a Raman endoscope on resection margins and ambiguous regions on the white light image to provide accurate guidance for additional excisions when required.
In terms of multiplicity, the early work by Zavaleta et al.
[32] remains one the most relevant example of multiplexed molecular imaging of cancer tissues with 10 different simultaneously discriminated SEP fingerprint. Nonetheless, the continuous development of sophisticated multivariate analysis techniques
[33] is enabling access to the complex molecular information contained in multidimensional datasets. At the same time, the database of potential SERS codes is progressively expanding, with the design of novel classes of superior molecular reporters with reduced spectral overlapping
[34] as well as the improvement of synthetic protocols for scalable fabrication of SEPs independently of the chemical nature of the SERS code
[35][36][35,36]. Thus, we expect that several tens of SEPs can be soon routinely implemented in multiplex imaging analysis.
The development of advanced instrumentation focusing at reducing the acquisition time for Raman mapping is currently progressing
[37]. However, scanning large areas of healthy tissues for identification of tumor spots with high spatial resolution still remains a major obstacle for the practical implementation of SERS imaging in the clinical practice. A relatively straightforward solution to such a low-throughput issue is offered by the designing of multimodal optical probe integrating fluorescent emitters into SEPs
[38][39][40][38,39,40]. For instance, covalent loading of organic fluorophores can be easily achieved within the silica matrix surrounding the plasmonic core
[41]. In this dual mode approach, fluorescence spectroscopy provides a first read-out for fast screening over large tissue areas while multiplexed SERS analysis is subsequently performed to precisely and quantitatively interrogate selected areas of interest.
Beyond imaging of tissues for discrimination of tumor vs healthy sites, SERS imaging with SEPs can be further extended to the analysis at the single cell level. In this scenario, the high multiplexing capability of SERS has the potential to provide accurate molecular phenotyping of the individual cancer cells. This entails the multidimensional characterization and discrimination of functionally relevant discrete cell sub-populations which are otherwise lost in population-averaged bulk measurements or not captured by genotypic studies. Notably, from static multiplex phenotyping of fixed samples
[42], SERS single-cell analysis is advancing towards dynamic, non-destructive interrogation of live cells. Real-time monitoring of single cancer cell molecular diversity, under different conditions and extracellular stimuli, is expected to address fundamental questions about tumor biology while helping at expanding the library of clinically relevant biomarkers for better diagnosis, risk stratification for treatment decisions, prognosis, and drug development
[1]. To this end, SERS spectroscopy is typically coupled with microfluidic technologies to facilitate single-cell manipulation and automated analysis
[43]. In particular, trapping methods that avoid physical contacts or application of forces that perturb the biological nature of the cell are more suited for dynamic interrogations of molecular biomarkers
[44]. An interesting combination of SERS and single-cell microfluidics has been recently reported by Willner et al.
[20]. A droplet microfluidic device has been designed to encapsulate single-prostate cancer cells, previously incubated with SEPs targeting sialic acid expressed on the cancerous cell membrane, and store them into a chamber array for subsequent optical interrogation (
Figure 1F). A data processing and analysis tool was developed to automatically perform and statistically analyze SERS mapping with tunable spectral resolution. Conceptually, the optical imaging is carried out in two steps. First, a large area of the chamber arrays is scanned with a low spectral resolution to rapidly identify areas of interest (
Figure 1G). In a second step, these selected spots are mapped at a higher spectral resolution, thereby permitting the dynamic monitoring of sialic acid distribution at the single-cell level in a higher throughput fashion (
Figure 1H).
The potential use of multiplex SERS imaging in dynamic single cell analysis is an extremely promising route for acquiring new insights into the mechanisms driving heterogeneity of cancer cells in tumors and the identification of new biomarkers. Nonetheless, it remains far less explored as compared to the identification and quantification of circulating tumor cells (CTCs), a topic that will be discussed in the next section.