Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Arthur Ouwehand and Version 2 by Peter Tang.
Xylitol has been widely documented to have dental health benefits, such as reducing the risk for dental caries. In skin, xylitol has been reported to improve barrier function and suppress the growth of potential skin pathogens.
- sugar alcohol
- prebiotic
- oral health
1. Introduction
Xylitol is a five-carbon sugar alcohol (C5H12O5, Figure 1) with a molecular weight of 152.15 g/mol, which is commonly used as a sweetener in sugar-free confectionery. It also naturally occurs in fruits and vegetables (plums, strawberries, cauliflower, and pumpkin[1]). It is equisweet to sucrose and has a very similar sweetness-time intensity to sucrose. Xylitol is the sweetest of all polyols[2]. Xylitol is best known for its dental benefits, such as reducing the risk for dental caries[3]. This is thought to function through three mechanisms: xylitol replaces cariogenic sucrose, xylitol may stimulate salivation, and xylitol may have specific inhibitory effects on Streptococcus mutans—the main causative microbe of dental caries[4]. Although a recent meta-analysis concluded that there is a need for high-quality studies on the dental benefits of xylitol, the same study concluded nevertheless that xylitol is an effective strategy as a self-applied caries preventive agent[3]. Furthermore, the European Food Safety Agency has approved a health claim “xylitol chewing gum reduces the risk of caries in children” [5]. Here, however, we want to focus on the potential health benefits of xylitol in skincare.
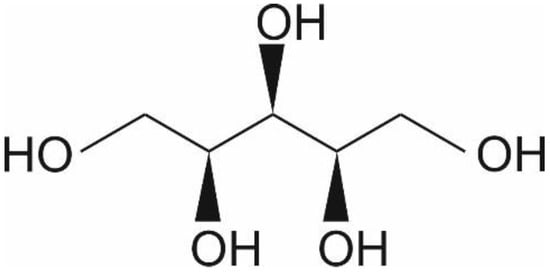
Figure 1.
Chemical structure of xylitol ©IFF Health & Biosciences.
Approximately half of the consumed xylitol is absorbed; the liver readily converts it to xylose by a non-specific cytoplasmic NAD-dependent dehydrogenase. The formed xylose is phosphorylated via a specific xylulokinase to xylulose-5-phosphate, an intermediate of the pentose-phosphate pathway before conversion to glucose, which is only slowly released into the bloodstream or stored as glycogen[6][7].
2. Skin Introduction
The skin acts as a barrier between the body and its surrounding environment. The epidermis is made up of the stratum corneum (outermost layer of the skin, Figure 2); formed by terminally differentiated epidermal keratinocytes and lipids, which play a main role as a physical and chemical permeability barrier. Under this lies the stratum granulosum, which forms a paracellular barrier that regulates the loss of moisture through the skin, as shown in Figure 2. Below that are the stratum spinosum, basal cells, and melanocytes, which are also part of the epidermis. The epidermal barrier, which is constantly being renewed, is characterized by its capacity to adapt to changing conditions in the environment[8]. The dermis, the next layer, supports the epidermis and produces matrix proteins such as elastin and collagen, as shown in Figure 2.
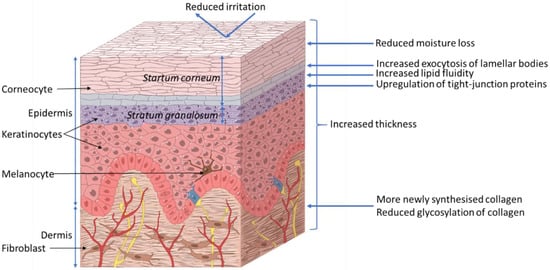

Figure 2.
Proposed effects of xylitol on skin health. ©Pinja Kettunen/SciArt & IFF Health & Biosciences.
3. Xylitol Benefits to Skin
Xylitol (100 mM) for 2 h has been observed, in an epidermal-equivalent skin model, to improve lipid fluidity in the uppermost layer of the stratum granulosum. The model consisted of normal human epidermal keratinocytes (NHEKs); isolated from donated skin samples; cultured ex vivo, and studied microscopically using lipid specific staining. The improved lipid fluidity accelerated the release of lipids and accelerates the exocytosis of lamellar bodies to the intercellular domain between stratum granulosum and stratum corneum thereby improving the lamellar structure and accelerating epidermal permeability barrier recovery[9]. Indeed, volunteers (n = 7) who had the inside of their forearms mechanically irritated by repeated tape stripping, were observed to have significantly less moisture loss; approximately 20%, when exposed to 100 mM xylitol for 10 min as compared to water. This was measurable both 1.5 and 2 h after exposure[9].
Further studies with NHEKs have shown that the viability and intracellular calcium concentration were not affected by 0.0045%–0.45% xylitol (calcium regulates keratinocyte differentiation) after 24 and 48 h as compared to the cell culture medium alone. However, xylitol up-regulated the expression of filaggrin, loricrin, involucrin, and occludin mRNA as measured by qPCR[10]. These proteins are involved in barrier function and tight junction (TJ) formation in the skin; occludin is the major protein in TJs, filaggrin or filament aggregating protein is a filament associated protein that binds keratin fibers in epithelial cells, loricrin is the major protein in cornified cells and contributes to barrier function of the skin, involucrin is bound to loricrin[11]. Moreover, 0.45% xylitol stimulated the mitogen-activated protein kinase (MAPK) pathway in the NHEKs and induced the activation-dependent translocation of protein kinase Cδ, after 48h as determined by Western blotting, a key promoter of epidermal differentiation[10]. The effect on the other cell types in the epidermis was not investigated in this model. Twelve healthy volunteers with dry skin received topical exposure to a combination of 5% glycerol and 5% xylitol for 14 days. This was observed to be associated with increased hydration, reduced moisture loss and increased dermal and epidermal thickness, as measured from biopsies and histological staining, compared to the untreated control arm of the same volunteer. In agreement with the above-described ex vivo keratinocyte studies, increased expression of filaggrin in epidermal cells in biopsies taken from the volunteers was also observed[12]. The separate contribution of xylitol and glycerol in the observed effects cannot be determined from this study.
In a study with hairless mice (23/group), skin irritation induced by 3 h topical application of 5% sodium dodecyl sulfate (SDS) was reduced with concomitant exposure to 8.26% xylitol or 5% glycerol (same osmolarity); transepidermal water loss was reduced and in the irritated area blood flow was reduced as well, as determined by videomicroscopy. Histological staining indicated that the epidermal thickness was increased in response to xylitol treatment compared to SDS alone[13]. Also in healthy adult volunteers (n = 16), the transepidermal water loss induced by experimental irritation with 0.1% SDS could be inhibited by simultaneous exposure for 24 h to 4.5% or 15% xylitol and 2.6% or 9.0% glycerol, but not 5.4% or 18% mannitol (same osmolarity) as compared to another site on the same arm with 0.1% SDS alone for 24 h[14]. These results suggest a polyol-specific response.
In a study with male rats, the inclusion of 10% xylitol to basic chow for 20 months was observed to be associated with a thicker skin and more acid-soluble collagen was observed, as determined from biopsies. Also, less collagen fluorescence was observed, which is a marker for collagen glycosylation and aging[15]. However, no difference in collagenase soluble and insoluble collagen was observed nor more total collagen as compared to control animals fed the same chow without xylitol[16]. Three months dietary supplementation with 10% xylitol in basic chow has been reported to increase the amounts of acid-soluble and total collagen (expressed as hydroxyproline) in the skin of streptozotocin-induced type 1 diabetic male rats (10 animals/group) as compared to type 1 diabetic animals fed unsupplemented chow. Also here, reduced hexose concentrations of acid-soluble collagen and reduce fluorescence of the collagenase-soluble fraction; indicating reduced glycosylation were observed. Similar observations on increased were made for non-diabetic rats (10 animals/group) after three months on 10% xylitol supplemented chow as compared to non-diabetic rats fed unsupplemented chow; for acid-soluble and total collagen, as well as reduced hexose concentrations of acid-soluble collagen and reduced fluorescence of the collagenase-soluble fraction in the skin[17].
The selective antimicrobial activity of xylitol, observed in dental health, has also been applied to wound care. In vitro studies with a Lubbock Chronic Wound Biofilm model have shown that the application of 2%, 10%, and 20% xylitol in water reduced growth Pseudomonas aeruginosa, Staphylococcus aureus, and Enterococcus faecalis compared to the water control. The highest concentration was observed to completely abolish biofilm formation[18] [19]. In a study with two strains of S. aureus and two strains of Staphylococcus epidermidis, xylitol alone or in combination with other prebiotics (galacto-oligosaccharides, fructo-oligosaccharides, isomalto-oligosaccharides, arabinogalactan, inulin or dextran) was able to reduce levels of planktonic bacteria and biofilm formation, in vitro[19]. Furthermore, another in vitro study showed that the combination of 5% xylitol and 2% lactoferrin could reduce the biofilm formation of P. aeruginosa and methicillin-resistant S. aureus after 72 h in a colony drip flow reactor, as compared to base wound dressing alone[20]. The anti-S. aureus potential of xylitol has also been investigated in human volunteers. Seventeen volunteers with atopic dermatitis received skin lotion with or without a combination of 5% xylitol and 0.2% farnesol on either arm for seven days. Compared to the control arm treated with unsupplemented lotion, S. aureus was significantly reduced, and skin moisture increased[21]. The contribution of xylitol alone cannot be deduced from this study. A further potential benefit of xylitol in wound care is the negative dissolution energy[2] which gives a cooling effect to the tissue.
4. Conclusions
Topical exposure of the skin with xylitol has thus been shown to reduce skin moisture loss. The mechanism appears to relate to increased tight junction and barrier formation in the skin. Also, dietary exposure to xylitol has been found to improve skin thickness. The antimicrobial activity against skin pathogens has been documented mainly in combination with other compounds and the contribution of xylitol to the observed effects needs to be determined. Furthermore, many of these results have been obtained in vitro and in animal models at relatively high doses (10% of the diet); their applicability to humans thus still needs to be confirmed.
References
- Salim Ur-Rehman; Zarina Mushtaq; Tahir Zahoor; Amir Jamil; Mian Anjum Murtaza; Xylitol: A Review on Bioproduction, Application, Health Benefits, and Related Safety Issues. Critical Reviews in Food Science and Nutrition 2013, 55, 1514-1528, 10.1080/10408398.2012.702288.
- Bond, M.; Dunning, N.. Xylitol.; Mitchel, H., Eds.; Blackwell Publishing: Oxford, 2006; pp. 295-324.
- Chandrashekar Janakiram; C. V. Deepan Kumar; Joe Joseph; Xylitol in preventing dental caries: A systematic review and meta-analyses. Journal of Natural Science, Biology and Medicine 2017, 8, 16-21, 10.4103/0976-9668.198344.
- Kauko K. Mäkinen; Gastrointestinal Disturbances Associated with the Consumption of Sugar Alcohols with Special Consideration of Xylitol: Scientific Review and Instructions for Dentists and Other Health-Care Professionals. International Journal of Dentistry 2016, 2016, 1-16, 10.1155/2016/5967907.
- European Food Safety Authority; Xylitol chewing gum/pastilles and reduction of the risk of tooth decay - Scientific substantiation of a health claim related to xylitol chewing gum/pastilles and reduction the risk of tooth decay pursuant to Article 14 of Regulation (EC) No 1924/2006 - Sc. EFSA Journal 2008, 6, 852, 10.2903/j.efsa.2008.852.
- Geoffrey Livesey; Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutrition Research Reviews 2003, 16, 163-191, 10.1079/nrr200371.
- Amo Kikuko; Hidekazu Arai; Uebanso Takashi; Makiko Fukaya; Megumi Koganei; Hajime Sasaki; Hironori Yamamoto; Yutaka Taketani; Eiji Takeda; Effects of xylitol on metabolic parameters and visceral fat accumulation. Journal of Clinical Biochemistry and Nutrition 2011, 49, 1-7, 10.3164/jcbn.10-111.
- R. Abdayem; M. Haftek; Barrière épidermique. Annales de Dermatologie et de Vénéréologie 2018, 145, 293-301, 10.1016/j.annder.2017.12.001.
- Yuki Umino; Sari Ipponjima; Mitsuhiro Denda; Modulation of lipid fluidity likely contributes to the fructose/xylitol-induced acceleration of epidermal permeability barrier recovery. Archives of Dermatological Research 2019, 311, 317-324, 10.1007/s00403-019-01905-0.
- Edit Páyer; Judit Szabó-Papp; Lídia Ambrus; Attila Gábor Szöllősi; Mónika Andrási; Shabtay Dikstein; Lajos Kemeny; Istvan Juhász; Andrea Szegedi; Tamás Bíró; et al.Attila Oláh Beyond the physico-chemical barrier: Glycerol and xylitol markedly yet differentially alter gene expression profiles and modify signalling pathways in human epidermal keratinocytes. Experimental Dermatology 2018, 27, 280-284, 10.1111/exd.13493.
- Nina Kirschner; Rita Rosenthal; Rothee Günzel; Ingrid Moll; Johanna M. Brandner; Tight junctions and differentiation - a chicken or the egg question?. Experimental Dermatology 2011, 21, 171-175, 10.1111/j.1600-0625.2011.01431.x.
- C Korponyai; E Szél; Z Behány; E Varga; G Mohos; Á Dura; S Dikstein; L Kemény; G Erős; Effects of Locally Applied Glycerol and Xylitol on the Hydration, Barrier Function and Morphological Parameters of the Skin. Acta Dermato Venereologica 2017, 97, 182-187, 10.2340/00015555-2493.
- E. Szél; H. Polyánka; K. Szabó; Petra Hartmann; D. Degovics; Németh István Balázs; I.B. Németh; C. Korponyai; E. Csányi; J. Kaszaki; et al.S. DiksteinK. NagyLajos KeményG. Erős Anti-irritant and anti-inflammatory effects of glycerol and xylitol in sodium lauryl sulphate-induced acute irritation. Journal of the European Academy of Dermatology and Venereology 2015, 29, 2333-2341, 10.1111/jdv.13225.
- Csilla Korponyai; Réka K. Kovács; Gábor Erös; Shabtay Dikstein; Lajos Kemény; Antiirritant Properties of Polyols and Amino Acids. Dermatitis 2011, 22, 141-146, 10.2310/6620.2011.10082.
- P.R. Odetti; A. Borgoglio; Ranieri Rolandi; Age-related increase of collagen fluorescence in human subcutaneous tissue. Metabolism 1992, 41, 655-658, 10.1016/0026-0495(92)90059-j.
- Pauli T. Mattila; Päivi Pelkonen; Matti L.E. Knuuttila; Effects of a Long-Term Dietary Xylitol Supplementation on Collagen Content and Fluorescence of the Skin in Aged Rats. Gerontology 2005, 51, 166-169, 10.1159/000083988.
- M.L.E. Knuuttila; T.H. Kuoksa; M.J. Svanberg; P.T. Mattila; K.M. Karjalainen; E. Kolehmainen; Effects of dietary xylitol on collagen content and glycosylation in healthy and diabetic rats.. Life Sciences 2000, 67, 283-290, 10.1016/s0024-3205(00)00621-4.
- Scot Dowd; Y. Sun; E. Smith; J.P. Kennedy; C.E. Jones; R. Wolcott; Effects of biofilm treatments on the multi-species Lubbock chronic wound biofilm model. Journal of Wound Care 2009, 18, 508-512, 10.12968/jowc.2009.18.12.45608.
- Silvia Di Lodovico; Franco Gasparri; Emanuela Di Campli; Paola Di Fermo; Simonetta D’Ercole; Luigina Cellini; Mara Di Giulio; Prebiotic Combinations Effects on the Colonization of Staphylococcal Skin Strains. Microorganisms 2020, 9, 37, 10.3390/microorganisms9010037.
- Mary Cloud B Ammons; Loren S Ward; Garth A James; Anti-biofilm efficacy of a lactoferrin/xylitol wound hydrogel used in combination with silver wound dressings. International Wound Journal 2011, 8, 268-273, 10.1111/j.1742-481x.2011.00781.x.
- Katsuyama Masako; Kobayashi Yusuke; Ichikawa Hideyuki; Mizuno Atsuko; Miyachi Yoshiki; Matsunaga Kayoko; Kawashima Makoto; A novel method to control the balance of skin microflora: Part 2. A study to assess the effect of a cream containing farnesol and xylitol on atopic dry skin. Journal of Dermatological Science 2005, 38, 207-213, 10.1016/j.jdermsci.2005.01.003.
More
