PDT has been approved by the United States Food and Drug Administration (FDA) to treat patients with non-small cell lung cancer, esophageal cancer, and Actinic Keratoses, as well as age-related macular degeneration. Clinical trials have also demonstrated PDT efficacy for mesothelioma, prostate, bladder, brain cancer, and head and neck cancers as well as bacterial, fungal, and viral infections. In many cases, photosensitizing agents are also used off label with noted successes by clinicians who are comfortable with photomedicine and laser light delivery.
- photodynamic therapy (PDT)
- photomedicine
- pancreatic ductal adenocarcinoma (PDAC)
- pancreatic cancer
- stroma
- combination therapy
- drug delivery
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is among the most lethal of human malignancies, with a 5-year survival rate of approximately 10% in the USA [1][2][1,2]. Surgery is possible in only 20% to 30% of patients and options are particularly limited for patients with unresectable disease [3]. Where resection is possible, the Whipple procedure, or pancreaticoduodenectomy is performed, in which the head of the pancreas, duodenum, gallbladder, and the bile duct are removed. This is a complex procedure with a significant impact on quality of life [4][5][4,5]. Virtually all clinical trials of chemotherapy drugs, targeted agents and combinations have failed to provide meaningful improvements in survival and most patients ultimately end up receiving palliative treatment [6][7][6,7].
For patients with advanced disease, palliative treatment has traditionally involved chemotherapy with either 5-fluorouracil (5-FU) or gemcitabine. In a clinical study reported in 1997, gemcitabine was found to impart a slight survival advantage (median = 5.65 months) compared with 5-FU (median = 4.41 months) and modest improvement in quality of life [8]. Since then, gemcitabine has become a mainstay for palliative care of advanced PDAC. More recently, a multidrug cocktail called FOLFIRINOX has been used, which is the combination of 5-FU with three other chemotherapy regimens (oxaliplatin, irinotecan, and leucovorin). Compared to gemcitabine, the FOLFIRINOX regimen achieves significant benefit in median survival of 11.1 months vs. 6.8 months for gemcitabine, though with significantly increased toxic effects, making it a viable option only for patients who are otherwise relatively healthy [9]. In a later study, survival benefit was observed to be further enhanced using a modified FOLFIRINOX combination [10]. Collectively, these results point to the fact that there remains an urgent need for strategies to overcome this disease.
2. PDT Mechanism and Clinical Implementation
When PS molecules absorb light, they undergo excitation from the ground state to an excited state depending on incident photon wavelength. Excited molecules rapidly drop back to their lowest vibrational level of the electronic excited state from which they can return back to the ground state either through non-radiative decay or by emitting photons with longer wavelength and lower energy (fluorescence). These processes have a short lifetime (in the order of nanoseconds) and do not lead to subsequent photochemistry. However, fluorescence emission from the PS, as a tumor-localizing fluorophore, is invaluable for imaging purposes as reviewed extensively elsewhere [11][20]. Another possibility is that the excited PS molecules undergo intersystem crossing to an excited triplet state. The lifetime of these states is long (in the order of microseconds or milliseconds) since the spin states are parallel instead of anti-parallel. Thus, it is forbidden for the PS molecules to go back to the ground state. Instead, they could either initiate photochemical reactions by transferring electrons to form reactive oxygen species (ROS) (type 1), or transfer their energy to the ground-state triplet oxygen molecule ( 3O 2) to give rise to singlet oxygen molecule ( 1O 2) through collisional quenching (type 2). These products are highly reactive and can cause cellular toxicity ( Figure 12 ) [11][12][20,21]. Although type 1 PSs are effective even in a hypoxic environment, all current clinically approved PSs, including those which have been studied for pancreatic cancer, impart toxicity primarily by the type 2 mechanism [13][22].
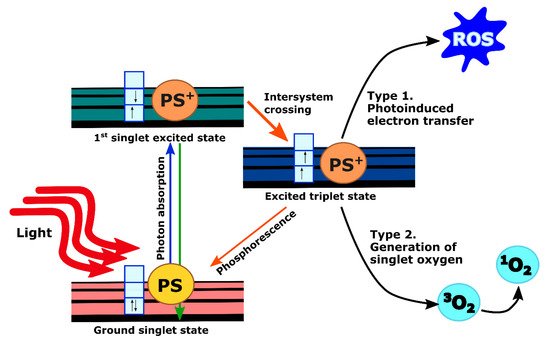
PDT does offer several inherent advantages. Depending on the localization of the PS, PDT can directly damage or alter targets in tumor cells. Additionally, since the visible or near infrared light used in PDT is non-ionizing, PDT does not carry the accumulating toxicity associated with radiotherapy [14][15][12,23]. However, unlike ionizing radiation, a noted challenge with PDT is the limited penetration of red and near-infrared wavelengths in tissue. Light delivery for internal sites such as the pancreas requires careful treatment planning and dosimetry, though innovative solutions have been developed and clinically validated as discussed further below.
3. Role of PDAC Stroma and Implications for PDT
PDAC is characterized by the development of a particularly dense fibrotic stroma, including cellular and non-cellular components such as pancreatic stellate cells (PSCs), which differentiate into heterogeneous fibroblastic cells, type I collagen, immune cells, adipocytes, and hyaluronan [16][35]. This complex microenvironment plays multiple roles in regulating tumor growth and response to therapy, and as discussed here, presents challenges and opportunities for PDT.
One important consequence of the profound desmoplastic reaction in PDAC is the impact of compressive stress from accumulated fibrotic stroma, not only on cancer cells, but on blood and lymphatic vessels as well ( Figure 24 ) [17][36]. The ill-functioning blood and lymphatic vessels limit drainage out of the tumor, causing elevated interstitial fluid pressure (IFP) [18][19][20][21][37,38,39,40]. As IFP increases to the value of microvascular pressure (MVP), the transportation of molecules to the tumor stops, which can lead to impermeability of large parts of PDAC tumor to therapeutic deliveries [22][41]. These hypo vascular tumors are also highly hypoxic, with oxygen percentage dropping from 7.5%, which is the estimated level in normal pancreas, to 0.3% in the pancreatic tumor [23][42]. While targeting tumor vasculature on one hand is a target for therapy, when vasculature is destroyed to cut off nutrition in cancer cells, it can also cause hypoxia through stimulation of several signaling pathways [24][25][26][27][28][29][43,44,45,46,47,48]. The role of hypoxia is further complicated in PDT, which requires oxygen and can also exacerbate hypoxia in tumors by consuming oxygen which is already present in order to produce ROS.
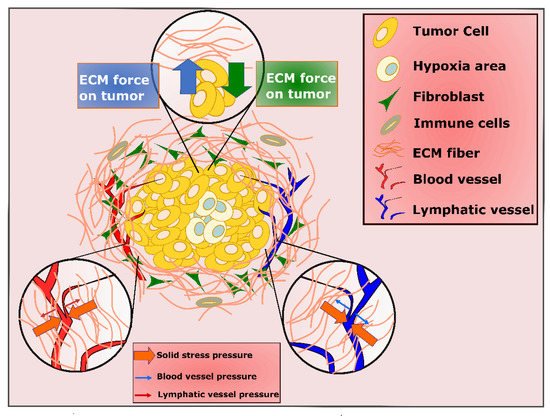
The challenge of tumor hypoxia has motivated efforts to design oxygen delivery/producing strategies to enhance PDT response in PDAC. As an example, there have been many studies utilizing oxygen-loaded microbubbles to deliver oxygen to the tumor microenvironment [30][31][32][49,50,51]. Additionally, since as cells progress toward malignancy, they become capable of producing excessive amount of H 2O 2, some studies have been focusing on in situ oxygen production by reaction between H 2O 2 and nanoparticles, nanorods, or catalase [33][34][35][52,53,54].
Moreover, it is important to know that both biophysical and biochemical properties ultimately impact on the fate of the tumor. Although the stiffness associated with PDAC desmoplasia can promote invasion and malignancy, biochemical interactions between ECM and PDAC cells can also regulate tumor growth and cell invasion [36][37][38][39][88,89,90,91]. For example, in 3D cell culture models, increased PDAC invasion has been observed in a softer environment (rich in collagen I) compared to a stiffer laminin-rich environment, underscoring the importance of both biochemical and mechanical properties of the ECM [40][41][92,93]. There is also a distinction between the activation of invasive behavior (which can be promoted by confinement and stiffness of surrounding material) and invasion itself, which requires enzymatic degradation (and hence softening) of ECM to enable invasive motility. Notably, in the same study, it was shown that populations of drug-resistant cells with increased invasiveness correlated with increased EMT were also more responsive to PDT [40][92]. This is consistent with a previous report which also showed increased EMT in drug-resistant PDAC cells [42][94].
4. PDT in Combination with Other Therapies
The combination of PDT with classical chemotherapy drugs has shown promise for synergy and to potentially reduce the chemotherapy dose and associated systemic toxicity [43][44][45][95,96,97]. Chemoresistance in cancer is associated in part with the hallmark characteristic of resisting cell death by increased antiapoptotic signaling [46][98]. PDT has been shown to target the anti-apoptotic proteins such as BCL-2, thus tipping the balance toward pro-apoptotic signaling and making cancer cells more responsive to chemotherapy [47][99]. Gemcitabine, although it only provides marginal survival benefit, has remained the primary treatment for advanced pancreatic cancer [44][48][96,100]. It has been shown that combination of PDT with low-dose gemcitabine significantly reduced the volume of the pancreatic tumor without any adverse effect in vivo [49][101]. One study investigated the possibility of enhancing oxaliplatin efficacy using benzoporphyrin derivative-mediated (BPD, verteporfin)–PDT in 3D culture models [50][102]. The results from this study indicate that the combination of these therapies was significantly more effective compared to either therapy alone. This was due to the distinct cytotoxic mechanisms of these therapies. While oxaliplatin induced DNA damage, BPD–PDT mainly targeted mitochondrial membrane and as a result lacked any overlap in toxicity of the chemotherapy agent. This is an important point to consider since it provides better disease management to healthy tissue. The study also showed that combining two mechanistically distinct therapies did not guarantee the enhancement in the therapy response and emphasized the need for physiologically relevant models to assess combinations. Similar treatment effects were observed in other cancer models that suggest in general PDT synergizes with platinum-based chemotherapies [51][52][53][54][103,104,105,106]. Moreover, PDT and chemotherapy not only have distinct mechanisms of action within the cell, but they also target different cells within the tumor. The results from a 3D model of PDAC showed that while chemotherapy was able to decrease the live volume of the spheroid, it had almost no effect on the invading cells. The same study showed that the opposite result was true for PDT, where invading cells died the most after treatment [40][92]. Additionally, it has been shown that PDT is able to disrupt adherent junctions between cancer cells [55][107]. This could be potentially significant if PDT is administrated prior to chemotherapy, enhancing paracellular drug transport into nodules, as well as by depleting surrounding stroma as discussed earlier. Furthermore, use of PDT as a pre-treatment in which the light-based production of ROS by design is not sufficient to surpass the cytotoxicity threshold but does elicit biological response. This approach, which has been termed photodynamic priming (PDP), has been shown to overcome chemotherapy resistance by combination with vitamin D3 receptor activation in the pancreatic tumor microenvironment (TME) [56][108].
In addition to interacting with classical chemotherapies, PDT may also play an inherently complementary role with other light-based approaches. Photothermal therapy (PTT) is a therapeutic technique that uses photothermal agents (usually gold) to convert the absorbed light into heat, which induces cancer cell death [57][58][109,110]. This method by itself or in combination with other therapies have been used for pancreatic cancer. The combination of PDT and PTT provides a promising strategy to enhance therapeutic efficiency in PDAC [59][111]. Although PTT by itself is unable to eliminate all cancer cells in the tumor due to constant heat lost by circulating blood, when combined with PDT, it can compensate (for the reduced efficacy) of oxygen-dependent PDT in the hypoxic tumor [60][61][112,113].
An inherent challenge in PDT treatment of solid tumors is the limitation of light penetration in biological tissue. Sonodynamic therapy (SDT) is another ROS-dependent strategy that works based on the synergistic interaction of drugs and ultrasound. Due to the relatively low tissue attenuation coefficient of ultrasound, it can penetrate deeply into the tissue [62][114]. Yet another ROS-dependent strategy is chemodynamic therapy (CDT), which works based on H 2O 2 conversion into toxic hydroxyl radicals (OH) and leads to cell apoptosis [63][115]. Combination of PDT and SDT or CDT are relatively new approaches that can overcome the hypoxia in PDAC and as a result enhance the generation of ROS in pancreatic cancer [64][65][66][116,117,118].
PDT is also conducive to combination with radiation therapy (RT), with which it shares similar dosimetry and overlapping communities of practitioners and medical physicists. For example, it has been shown that Cerenkov radiation produced by high-energy X-rays passing through tissue can activate PS [67][68][69][119,120,121]. Photosensitizers for PDT may also serve as radiosensitizers, potentially providing improved radiation delivery to target tissue while reducing overall radiation dose. The combination of PDT and RT has also been explored through the innovative combination of radioluminescent nanoparticles which are excited during RT with deep-tissue-penetrating X-rays, producing luminescence which in turn activates conjugated PS to achieve a low-dose PDT effect in deep tissue [70][122]. A recent study on 3D pancreatic cancer coculture models investigated whether PDT synergizes with RT when combined in the absence of nanoparticles and showed the beneficial effect of PDT on RT efficacy [71][123]. This study suggests the potential of nanoscintillator-induced PDT as another strategy for deep-tissue treatment, where both therapies are simultaneously activated by the ionizing radiations.
