Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Daniela Pasero.
It is known that bacterial infections represent a common complication during viral respiratory tract infections such as influenza, with a concomitant increase in morbidity and mortality. Nevertheless, the prevalence of bacterial co-infections and secondary infections in critically ill patients affected by coronavirus disease 2019 (COVID-19) is not well understood yet. We performed a review of the literature currently available to examine the incidence of bacterial secondary infections acquired during hospital stay and the risk factors associated with multidrug resistance.
- hospital acquired infection
- multi-drug resistance
- COVID-19
- SARS-CoV-2
- secondary bacterial infections
- critically ill patients
1. Introduction
Whether critically ill patients affected by coronavirus disease 2019 (COVID-19) are at higher risk of bacterial infections has already been a matter under discussion. The main concern is related to the time of infection development, whether early at admission with SARS-CoV-2 infection or later during the intensive care unit (ICU) stay, which is indeed per se a risk factor of developing hospital acquired infections. Bacterial infections are commonly reported at admission in cases of viral respiratory tract infection such as influenza and its incidence is highly variable: around 20–30% of patients are diagnosed with severe influenza [1,2,3][1][2][3]. The coexistence of viral and bacterial infections at admission is usually associated with greater severity of illness and increased risk of death [4]. Therefore, both the severity of the respiratory disease in patients affected by COVID-19 at ICU admission and the difficulty of ruling out a bacterial co-infection on presentation lead to a wide prescription of broad-spectrum antimicrobial drugs. Moreover, the extensive introduction of empiric antimicrobial therapy was endorsed by the Surviving Sepsis Campaign guidelines, which suggested the use of empiric antimicrobial treatment over no treatment at ICU admission in ventilated patients [5]. Several retrospective analyses reported a quite low incidence of bacterial infections at admission compared to previous epidemic viral infections, such as two different meta-analyses that reported an incidence of co-infections between 3.5% (95%CI 0.4–6.7%) and 14% (95% CI 5–26%) in the ICU context; therefore, the prescription of antimicrobial therapy should be reduced and carefully evaluated only for specific cases [6,7][6][7]. However, many patients affected by COVID-19 developed hospital acquired infections during their ICU stay, further increasing the severity of organ dysfunction, especially in cases complicated by septic shock that showed doubling mortality rates [8]. COVID-19 can be associated with a significant and sustained lymphopenia compromising the immune system; in fact, a significant decrease in lymphocyte and increase in neutrophil count has been described, especially in the most severe cases, which presented with an inflammatory storm. The overall incidence of bacterial infections in patients admitted with a diagnosis of COVID-19 has been estimated at 6.9% (95%CI 4.3–9.5%), while the incidence increased to 8.1% (95% CI 2.3–13.8%) in critically ill patients [6[6][7],7], who are ventilator-dependent and need invasive monitoring and pharmacological support by central lines [9]. However, a small proportion of bacterial infections were described as co-infection, defined as those reported at the time of COVID-19 diagnosis, and existing at patient’s admission (3.5%, 95% CI 0.4–6.7%). Most of these infections were caused by community-acquired pathogens such as Streptococcus pneumoniae and Haemophilus influenzae, as it had already been described during the previous viral epidemic infections [4,6,7][4][6][7]. Most bacterial infections described in the COVID-19 population emerged during the ICU and hospital stay, were defined as secondary bacterial infections and their incidence was estimated around 14.3% (95% CI 9.6–18.9%) [6], as shown in Figure 1.
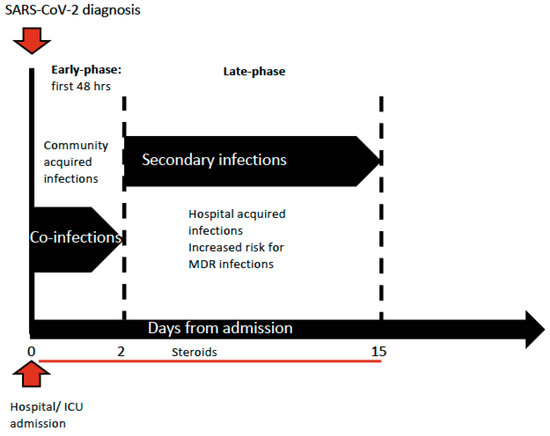
Figure 1. Timing of infections’ development during ICU stay.
2. Multidrug Resistance Organisms: Development and Spread during the COVID-19 Outbreak
The extensive use of broad-spectrum antimicrobial drugs during the COVID-19 outbreak might have contributed to the selection of pathogens with different profiles of resistance. All microorganisms such as Staphylococcus aureus, Enterococcus spp., Enterobacteriaceae, Pseudomonas aeruginosa and Acinetobacter spp. are often responsible for healthcare associated infections and prone to develop multidrug resistance. According to definitions of the European Center for Disease Prevention and Control (ECDC) and of the Center for Disease Control and Prevention (CDC), MDR infection is described as an acquired infection non-susceptible to at least one agent in three or more antimicrobial categories [44][10]. An incidence of MDR infections in critically ill COVID-19 patients ranging between 32% to 50% was reported by several papers [8,32,35,36,38,45][8][11][12][13][14][15]. A group of Italian authors tried to explain the possible mechanisms that led to an increase of multi-drug resistant strains among common bacteria, responsible for secondary infections in the COVID ICU, in a country where the spread of antibiotic resistant bacteria was very high and largely attributed to carbapenem resistant Klebsiella pneumoniae (CR-Kp) [46][16]. They retrospectively analyzed all microbiological samples of rectal swabs collected for routine surveillance before and during the pandemic to compare CPE colonization incidence. The authors observed an increase of CPE colonization up to 50% in patients admitted to their ICU with COVID-19. Interestingly, they observed that patients who underwent prone-supine position had higher incidence of CPE transmission compared to those who did not need that type of treatment, which involved several health care workers (HCW) each time the maneuver had to be performed. Moreover, they observed that the use of personal protection equipment (PPE) to protect HCW from SARS-CoV-2 transmission and the need to employ a larger number of HCW, who were not trained for intensive care medicine, might have reduced the awareness of hand hygiene, contact precautions and PPE disinfection when moving from one patient to another; the latter were regular practices described in an antimicrobial stewardship program (ASP) implemented in their ICU before the outbreak and they were in accordance with the ESCMID guidelines for the management of infection control measures to reduce transmission of multidrug resistant Gram-negative bacteria in hospitalized patients [47][17]. Furthermore, work overload of ICU staff, ICU overcrowding, reinforcement of less experimented staff and PPE shortage were described as potential additional factors leading to a decrease in adherence to infection control measurements, allowing the spread of MDROs [48,49,50][18][19][20]. Additionally, other authors reported a PPE shortage such as gowns with the need to use the same for more than one patient [36][13].
3. MDR Secondary Infections and Risk Factors
Most of the published studies on COVID-19 patients with complications of secondary infections were retrospective and reported single-centered data on a small number of patients, but the incidence and the time of development of the secondary infections were almost homogeneous. Karulli A. et al. reported 16 out of 32 patients developing an MDR infection with the prevalent isolates of CPE, and the highest mortality rate of 72%. Although the sample size was small, some considerations might be made about the high number of patients on empirical antibiotic therapy at admission (78%) and the high percentage of patients that were on steroids (53%) [32][11]. Similarly, Patel A. et al. reported a spread of MDR secondary infections caused by Gram negative bacteria; the whole sample of patients described had an MDR infection and the majority of infections reported were caused by CPE (46%). Moreover, nearly the whole studied population was on an empiric antibiotic therapy upon admission (97%) compared to the study by Karulli, and 35% of them received steroids [33][21]. Baiou A. et al. reported 33% of MDR secondary infections with around 10% of the isolated microorganisms as carbapenem-resistant Klebsiella pneumoniae and Pseudomonas aeruginosa. In the latter, differently from the two previous studies, patients were younger and few of them received steroids (4.7%). The only risk factor independently associated with MDR secondary infections was the invasive mechanical ventilation (OR 1.062; 95% CI 1.012–1.114) [34][22]. A reduction of antibiotic prescriptions has been described between January 2020 and April 2020 (OR 0.28, 95% CI 0.08–0.98) and a lower prescription was also observed in studies evaluating children (38.5%, 95% CI 26.3%–52.3%) when compared to studies including only adult population (83.4%, 95% C.I. 76.6–88.5%), while a higher antibiotic administration was described with the increasing of median or mean patient age, per 10 years increase (OR 1.45, 95 CI 1.18–1.77) and in patients requiring invasive mechanical ventilation (OR 1.33, 95% CI 1.15–1.54), with a 10% increase [51][23]. Cultrera R. et al. reported the microbiological characteristics of a high number of isolates in secondary infections on a small sample of patients: the incidence of MDR-acquired infections was similar to other retrospective studies. They found a higher frequency of methicillin-resistant Staphylococcus Aureus (MRSA), vancomycin-resistant Enterococcus faecium and carbapenem resistant Acinetobacter baumannii [35][12]. Bogossian E.G. et al. compared the incidence of multi-drug resistant organisms (MDRO) between patients admitted with COVID-19 and a control group of patients with subarachnoid hemorrhage (SAH), complicated by sepsis, admitted in a period before the outbreak. The incidence and characteristics of MDR secondary infections reported were similar to most of the data coming from the published articles on COVID-19 and did not show any significant difference in the multivariable competing risk analysis (sHR 1.71 (CI95% 0.93–3.21)) between the two groups; therefore, they concluded that COVID-19 was not an independent risk factor associated with MDR infections acquisition [36][13]. Nasir N. et al. reported that patients who were severe to critically ill at the time of admission with COVID-19 had an over fourfold probability to develop secondary infections (OR 4.42; 95% CI 1.63–11.9). In fact, the majority of those patients needed more invasive devices such as an endotracheal tube and central venous catheter; undergoing more frequent to invasive mechanical ventilation and, as a consequence, they were more frequently admitted to the ICU compared to the controls. Moreover, the authors showed that all patients developing secondary infections received the empiric antibiotic therapy at admission and an elevated percentage (92%) were on steroids treatment, which was an independent risk factor strongly associated with secondary infections (OR 4.6; 95% CI 1.24–17.05) [37][24]. Giacobbe D.R. et al. and Bonazzetti C. et al. both reported data on the incidence of blood stream infections (BSI) in critically ill COVID-19 patients [38,39][14][25]. The patients described in both papers were similar in the number of cases and age, but Bonazzetti C. et al. reported a higher number of MDR infections, caused especially by Enterococcus spp., with a higher rate of mortality (49% vs. 26%). Moreover, they observed that patients who developed BSI during their ICU stay had a higher sequential organ failure assessment (SOFA) score (9.5, IQR 8–12 vs. 8, IQR 5–10), but they did not report whether their patients were on an empirical antimicrobial therapy at ICU admission and if they received steroids [39][25]. Instead, Giacobbe D.R. et al. reported that 96% of their patient sample were on an empiric antimicrobial treatment at ICU admission and 31% received steroids: they found that the anti-inflammatory treatment was an independent risk factor for BSI development (caused-specific hazard ratio [csHR] 3.95; 95% CI 1.20–13.03 for methylprednisolone and csHR 10.69; 95% CI 2.71–42.17, for methylprednisolone plus tocilizumab) [38][14]. Interestingly, Luxemburger H. et al. reported that the use of proton pump inhibitors (PPI) (OR 2.37; 95% CI 0.08–5.22) and gastroesophageal reflux disease (OR 6.40; 95% CI 1.50–35.51) were independent predictive factors of developing secondary infections in patients affected by COVID-19. These results underlined the role of microaspiration in the pathogenesis of secondary bacterial infections of the lower respiratory tract [14][26]. Furthermore, a short term and current use of PPI during admission for COVID-19 increased the worst-outcome risk (OR 1.90; 95% CI 1.46–2.77 and OR 1.79; 95% CI 1.30–3.10) due to an increased probability of ICU admission, invasive mechanical ventilation, and death [52][27]. A recent meta-analysis confirmed such findings on a bigger population coming from seven papers (OR 1.46, 95% CI 1.34–1.60) [53][28]. Ripa et al. reported data on both BSI and low respiratory tract infections (pLRTI) among 86/731 (11.8%) admitted to the ICU [40][29]. Critically ill patients more frequently developed a secondary infection. During the follow-up of 1,318 patient-days (PDFUs), 40/731 patients (5.5%) had 51 secondary infections during ICU stay with an incidence rate of 38.7 (28.8–50.9) per 1000 PDFUs, which was considerably higher when compared to patients outside the ICU, with an incidence rate of 4.0 (2.9–5.5) per 1000 PDFUs. Among factors associated with an increased risk for secondary infections, there was ICU admission within the first 48 h from hospital admission (HR 2.51, 95% CI 1.04–6.05), together with decreasing PaO2/FiO2 ratio < 100 (HR 3.67; 95% CI 1.24–10.90). The results confirmed the median time for the first secondary BSI at 13 days from hospital admission with a prevalence of Gram-positive among the isolates (Staphylococcus epidermidis and Enterococcus faecium), while at 16 days for the first pLRTI, mainly caused by Gram-negative MDRO (carbapenem-resistant Acinetobacter baumannii, CP-Kp, CPE and carbapenem-resistant Pseudomonas aeruginosa). The authors observed an increase of antimicrobial resistance compared with an historical cohort of patients before the SARS-CoV-2 outbreak, with a higher incidence rate of Gram negative MDRO among BSIs, mainly due to Acinetobacter baumannii, while among Gram-positive isolates there was an increase of vancomycin-resistant Enterococcus faecium.
Only two multicenter retrospective studies reported the incidence and risk factors for secondary infections in critically ill patients [8,41][8][30]. The former, published by Grasselli G. et al. evaluated patients with severe COVID-19 admitted to eight Italian hub hospitals [8]. They reported data on a sample of 774 patients, in which 35% developed MDR infections, among 359 patients (46%) that developed hospital acquired infections (HAI). The most frequent secondary infections were ventilator associated pneumonia (VAP, N = 389 [50%]), with 26 (95% CI 23.6–28.8) per 1000 patient-days of invasive mechanical ventilation, and BSI (N = 183 [34%]), with 11.7 (95% CI 10.1–13.5) per 1000 ICU patient-days. They observed an incidence of MDR infections like most of the single center, and in the majority of the cases the isolated microorganisms were Enterobacterales with resistance to carbapenems and MRSA, especially in VAP. The number of patients that were on empirical antibiotic therapy was similar to the other single-center studies (69%), but nearly half of these patients received broad spectrum drugs. Notably, at the multivariable analysis they showed that treatment with broad spectrum antibiotics was independently associated with HAI (HR 0.61; 95% CI, 0.44–0.84), which paradoxically described a protective role of antimicrobial treatment against the development of secondary infections; however, they reported that the policy of all participating ICUs was to interrupt empiric antimicrobial therapy early if culture results were negative for bacterial infections at admission. In addition, the authors observed that patients complicated with septic shock resulted in a doubled mortality rate (52%). The other multicenter study, published by Giacobbe D.R. et al. involved nine Italian hub hospitals that admitted 586 patients with severe COVID-19 during the first wave of the outbreak between February and May 2020. Among them, 171 (29%) patients developed an infection with diagnosis of VAP. The incidence rate of VAP was slightly lower when compared to the study by Grasselli et al. (18 vs. 26 events per 1000 ventilator days). However, the incidence of MDR infections was similar and very close to all the other studies that reported this data. The most frequently isolated germs were Pseudomonas aeruginosa (35%), Staphylococcus aureus (23%) and Klebsiella pneumoniae (19%). Among the MDR microorganisms, the most frequently isolated species were methicillin-resistant S. aureus (10%) and carbapenem-resistant Gram-negative bacteria (32%). They expressed the outcome of their population at 30 days as a 30-day case-fatality and it was quite high (78/171, 46%), but it was evaluated only on patients who developed a secondary infection. Other studies reported the mortality rate over the whole COVID-19 population; therefore, no conclusion could be drawn comparing the outcome of the results among the selected studies. Interestingly, the authors found that septic shock and ARDS at VAP onset were strongly associated with an increase of 30-day case-fatality (OR 3.30; 95% CI 1.43–7.61 and OR 13.21; 95% CI 3.05–57.26 respectively). This association was confirmed when they analyzed the subgroup of patients who developed VAP with the BALF culture positive. The incidence rate of VAP reported in patients for both studies was surprisingly high, especially when compared to that related to non-COVID-19 critically ill patients (one out of 19 episodes per 1000 ventilator days) [54,55][31][32]. The increase of VAP incidence rate in COVID-19 critically ill patients might be explained by multiple triggering mechanisms: (i) one of the main hypothesis might be that the viral infection causes a direct damage of the lower airway epithelial cells associated with impaired mucociliary clearance, which allows bacterial binding to cell surfaces [10][33]; (ii) a second hypothesis might be an up-regulation of the airway cell receptors [10][33]; (iii) a third reason might be a decreased immune response or the abnormal release of inflammatory mediators during the early stages [11,12][34][35]. Furthermore, some authors speculated on the hypothesis that there might have been an overestimation during the COVID-19 period because in the majority of cases the diagnosis was clinical and performing a BALF was rare, especially at the beginning of the outbreak. This was due to the high risk of viral transmission from the patient to the operator despite the presence of PPE, as in several hospitals in Northern Italy where a shortage was reported during the first wave.
Lastly, Li J. et al. reported 102 (6.8%) who developed secondary bacterial infections among COVID-19 patients. Similarly to most of the selected studies, they observed that critically ill patients were more prone to develop secondary bacterial infections because they received invasive mechanical ventilation and had a higher rate of central catheter placement. Among patients who developed secondary infections, 69 cases (4.6%) were caused by MDRO, with carbapenem resistant Acinetobacter baumannii and Klebsiella pneumoniae as the most frequently isolated infection found mainly in critically ill patients. Almost half of the patients who acquired secondary infections died and the critically ill patients showed an increased mortality rate 45/69 (65%) compared to less severe patients, 5/33 (15.2%) [42][36].
References
- Klein, E.Y.; Monteforte, B.; Gupta, A.; Jiang, W.; May, L.; Hsieh, Y.H.; Dugas, A. The frequency of influenza and bacterial coinfection: A systematic review and meta-analysis. Influenza Other Respir. Viruses 2016, 10, 394–403.
- Rice, T.W.; Rubinson, L.; Uyeki, T.M.; Vaughn, F.L.; John, B.B.; Miller, R.R., 3rd; Higgs, E.; Randolph, A.G.; Smoot, B.E.; Thompson, B.T.; et al. Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit. Care Med. 2012, 40, 1487–1498.
- Shah, N.S.; Greenberg, J.A.; McNulty, M.C.; Gregg, K.S.; Riddell, J.t.; Mangino, J.E.; Weber, D.M.; Hebert, C.L.; Marzec, N.S.; Barron, M.A.; et al. Bacterial and viral co-infections complicating severe influenza: Incidence and impact among 507 U.S. patients, 2013–2014. J. Clin. Virol. 2016, 80, 12–19.
- Martin-Loeches, I.; Sanchez-Corral, A.; Diaz, E.; Granada, R.M.; Zaragoza, R.; Villavicencio, C.; Albaya, A.; Cerda, E.; Catalan, R.M.; Luque, P.; et al. Community-acquired respiratory coinfection in critically ill patients with pandemic 2009 influenza A(H1N1) virus. Chest 2011, 139, 555–562.
- Alhazzani, W.; Moller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020, 46, 854–887.
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629.
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275.
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients with COVID-19. Chest 2021, 160, 454–465.
- Nelson, J.E.; Cox, C.E.; Hope, A.A.; Carson, S.S. Chronic critical illness. Am. J. Respir. Crit. Care Med. 2010, 182, 446–454.
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281.
- Karruli, A.; Boccia, F.; Gagliardi, M.; Patauner, F.; Ursi, M.P.; Sommese, P.; De Rosa, R.; Murino, P.; Ruocco, G.; Corcione, A.; et al. Multidrug-Resistant Infections and Outcome of Critically Ill Patients with Coronavirus Disease 2019: A Single Center Experience. Microb. Drug Resist. 2021.
- Cultrera, R.; Barozzi, A.; Libanore, M.; Marangoni, E.; Pora, R.; Quarta, B.; Spadaro, S.; Ragazzi, R.; Marra, A.; Segala, D.; et al. Co-Infections in Critically Ill Patients with or without COVID-19: A Comparison of Clinical Microbial Culture Findings. Int. J. Environ. Res. Public Health 2021, 18, 4358.
- Bogossian, E.G.; Taccone, F.S.; Izzi, A.; Yin, N.; Garufi, A.; Hublet, S.; Njimi, H.; Ego, A.; Gorham, J.; Byl, B.; et al. The Acquisition of Multidrug-Resistant Bacteria in Patients Admitted to COVID-19 Intensive Care Units: A Monocentric Retrospective Case Control Study. Microorganisms 2020, 8, 1821.
- Giacobbe, D.R.; Battaglini, D.; Ball, L.; Brunetti, I.; Bruzzone, B.; Codda, G.; Crea, F.; De Maria, A.; Dentone, C.; Di Biagio, A.; et al. Bloodstream infections in critically ill patients with COVID-19. Eur. J. Clin. Investig. 2020, 50, e13319.
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-Garcia, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88.
- Tiri, B.; Sensi, E.; Marsiliani, V.; Cantarini, M.; Priante, G.; Vernelli, C.; Martella, L.A.; Costantini, M.; Mariottini, A.; Andreani, P.; et al. Antimicrobial Stewardship Program, COVID-19, and Infection Control: Spread of Carbapenem-Resistant Klebsiella Pneumoniae Colonization in ICU COVID-19 Patients. What Did Not Work? J. Clin. Med. 2020, 9, 2744.
- Tacconelli, E.; Cataldo, M.A.; Dancer, S.J.; De Angelis, G.; Falcone, M.; Frank, U.; Kahlmeter, G.; Pan, A.; Petrosillo, N.; Rodriguez-Bano, J.; et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin. Microbiol. Infect. 2014, 20 (Suppl. S1), 1–55.
- Clancy, C.J.; Buehrle, D.J.; Nguyen, M.H. PRO: The COVID-19 pandemic will result in increased antimicrobial resistance rates. JAC Antimicrob. Resist. 2020, 2, dlaa049.
- Ranney, M.L.; Griffeth, V.; Jha, A.K. Critical Supply Shortages—The Need for Ventilators and Personal Protective Equipment during the COVID-19 Pandemic. N. Engl. J. Med. 2020, 382, e41.
- Murray, A.K. The Novel Coronavirus COVID-19 Outbreak: Global Implications for Antimicrobial Resistance. Front. Microbiol. 2020, 11, 1020.
- Patel, A.; Emerick, M.; Cabunoc, M.K.; Williams, M.H.; Preas, M.A.; Schrank, G.; Rabinowitz, R.; Luethy, P.; Johnson, J.K.; Leekha, S. Rapid Spread and Control of Multidrug-Resistant Gram-Negative Bacteria in COVID-19 Patient Care Units. Emerg. Infect. Dis. 2021, 27, 1234–1237.
- Baiou, A.; Elbuzidi, A.A.; Bakdach, D.; Zaqout, A.; Alarbi, K.M.; Bintaher, A.A.; Ali, M.M.B.; Elarabi, A.M.; Ali, G.A.M.; Daghfal, J.; et al. Clinical characteristics and risk factors for the isolation of multi-drug-resistant Gram-negative bacteria from critically ill patients with COVID-19. J. Hosp. Infect. 2021, 110, 165–171.
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531.
- Nasir, N.; Rehman, F.; Omair, S.F. Risk factors for bacterial infections in patients with moderate to severe COVID-19: A case-control study. J. Med. Virol. 2021, 93, 4564–4569.
- Bonazzetti, C.; Morena, V.; Giacomelli, A.; Oreni, L.; Casalini, G.; Galimberti, L.R.; Bolis, M.; Rimoldi, M.; Ballone, E.; Colombo, R.; et al. Unexpectedly High Frequency of Enterococcal Bloodstream Infections in Coronavirus Disease 2019 Patients Admitted to an Italian ICU: An Observational Study. Crit. Care Med. 2021, 49, e31–e40.
- Luxenburger, H.; Sturm, L.; Biever, P.; Rieg, S.; Duerschmied, D.; Schultheiss, M.; Neumann-Haefelin, C.; Thimme, R.; Bettinger, D. Treatment with proton pump inhibitors increases the risk of secondary infections and ARDS in hospitalized patients with COVID-19: Coincidence or underestimated risk factor? J. Intern. Med. 2021, 289, 121–124.
- Lee, S.W.; Ha, E.K.; Yeniova, A.O.; Moon, S.Y.; Kim, S.Y.; Koh, H.Y.; Yang, J.M.; Jeong, S.J.; Moon, S.J.; Cho, J.Y.; et al. Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: A nationwide cohort study with propensity score matching. Gut 2021, 70, 76–84.
- Kow, C.S.; Hasan, S.S. Use of proton pump inhibitors and risk of adverse clinical outcomes from COVID-19: A meta-analysis. J. Intern. Med. 2021, 289, 125–128.
- Ripa, M.; Galli, L.; Poli, A.; Oltolini, C.; Spagnuolo, V.; Mastrangelo, A.; Muccini, C.; Monti, G.; De Luca, G.; Landoni, G.; et al. Secondary infections in patients hospitalized with COVID-19: Incidence and predictive factors. Clin. Microbiol. Infect. 2021, 27, 451–457.
- Giacobbe, D.R.; Battaglini, D.; Enrile, E.M.; Dentone, C.; Vena, A.; Robba, C.; Ball, L.; Bartoletti, M.; Coloretti, I.; Di Bella, S.; et al. Incidence and Prognosis of Ventilator-Associated Pneumonia in Critically Ill Patients with COVID-19: A Multicenter Study. J. Clin. Med. 2021, 10, 555.
- Bonell, A.; Azarrafiy, R.; Huong, V.T.L.; Viet, T.L.; Phu, V.D.; Dat, V.Q.; Wertheim, H.; van Doorn, H.R.; Lewycka, S.; Nadjm, B. A Systematic Review and Meta-analysis of Ventilator-associated Pneumonia in Adults in Asia: An Analysis of National Income Level on Incidence and Etiology. Clin. Infect. Dis. 2019, 68, 511–518.
- Dudeck, M.A.; Horan, T.C.; Peterson, K.D.; Allen-Bridson, K.; Morrell, G.; Anttila, A.; Pollock, D.A.; Edwards, J.R. National Healthcare Safety Network report, data summary for 2011, device-associated module. Am. J. Infect. Control 2013, 41, 286–300.
- Zhu, N.; Wang, W.; Liu, Z.; Liang, C.; Wang, W.; Ye, F.; Huang, B.; Zhao, L.; Wang, H.; Zhou, W.; et al. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat. Commun. 2020, 11, 3910.
- Chertow, D.S.; Memoli, M.J. Bacterial coinfection in influenza: A grand rounds review. JAMA 2013, 309, 275–282.
- McCullers, J.A. Insights into the interaction between influenza virus and pneumococcus. Clin. Microbiol. Rev. 2006, 19, 571–582.
- Li, J.; Wang, J.; Yang, Y.; Cai, P.; Cao, J.; Cai, X.; Zhang, Y. Etiology and antimicrobial resistance of secondary bacterial infections in patients hospitalized with COVID-19 in Wuhan, China: A retrospective analysis. Antimicrob. Resist. Infect. Control 2020, 9, 153.
More
