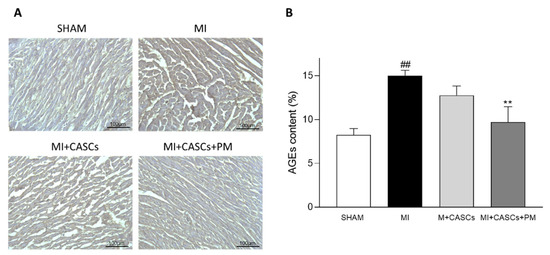
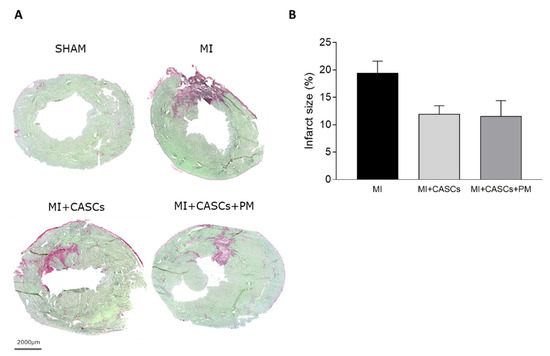
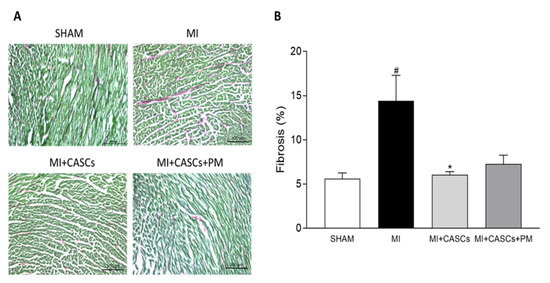
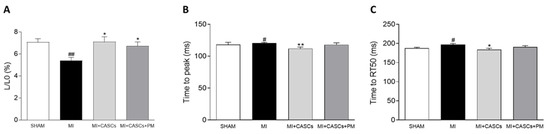
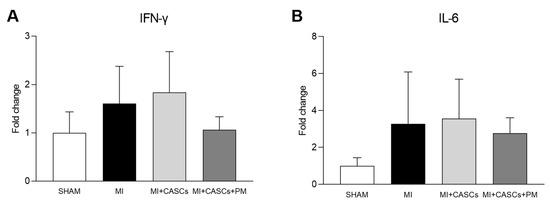
Independent of PM treatment, our data confirm that transplantation of CASCs can prevent worsening of cardiac function after an ischemic injury, as shown by Fanton et al. in the minipig model
[2][19]. Indeed, we have shown that EF significantly increased up to 20% after CASCs transplantation compared with non-treated animals. Meta-analysis of other CSCs therapies for the treatment of MI in mice showed an overall increase in EF of 9.9%
[29][46]. Therefore, CASCs do have more effective regenerative effects compared with other CSCs and are remarkable candidates for cellular therapy. Other parameters of global cardiac function, such as dP/dt
max, SV, CO, and ESV, even if not significantly affected, followed the same trend, indicating an overall improvement of systolic function upon CASCs transplantation. In addition, infarct size tended to decrease after CASCs transplantation. Furthermore, as shown by the prevention of adverse remodeling at the cardiomyocyte level, our data suggest that mechanical load subjected to the resident myocytes of the ischemic area was reduced with CASCs transplantation. The prevention of collagen deposition seen in our study is also in line with a potentially reduced mechanical load with CASCs transplantation. Indeed, an important pathway in post-MI remodeling and scar formation is the TGF-β1 signaling pathway. In this study, we did not evaluate the underlying mechanisms resulting in reduced fibrosis with CASCs. However, TGF-β1 could be an essential contributor. Indeed, increased TGF-β1 is detected after MI and is known to decrease the expression and function of enzymes responsible for matrix degradation and increase the inhibitors of proteases
[30][31][47,48]. Whether CASCs transplantation results in a reduced lysyl oxidase expression and/or PI3K/Akt, Smad3, and MAPK signaling pathway as a consequence of increased TGF-β1 activation
[32][49], remains to be confirmed. However, other studies have shown that MSCs transplantation is able to ameliorate cardiac fibrosis by decreasing TGF-β1 levels
[33][50]. Therefore, it seems likely that this TGF-β1 pathway is also involved in our study as it is a common pathway found in many diseases, but this has to be confirmed. In addition, we have demonstrated that CASCs transplantation can prevent adverse cellular remodeling of resident cardiomyocytes, isolated from the border zone of the infarct. Indeed, we show that, compared with MI animals, the amplitude and kinetics of cardiomyocyte shortening isolated from the border zone of MI in transplanted animals are improved. In that context, it has been described that the extent of mechanical load determines the extent of remodeling in both peri-infarct and remote regions
[34][51]. It is then very likely that even a small decrease in infarct size, which we observe upon CASCs transplantation, will reduce adverse cellular remodeling in the resident cardiomyocytes.
However, whether the beneficial cardiac outcome is solely attributed to new cardiomyocytes differentiated from CASCs or to the paracrine factors secreted by these stem cells remains to be investigated. This then raises the question of whether CASCs were still present 4 weeks after transplantation. Indeed, pre-clinical studies have shown that most of the stem cells injected at the site of injury are cleared out within seconds, resulting in only 1–3% of the injected cells persisting at the site of injury
[35][52]. Yet, previously, the presence of differentiated CASCs 8 weeks post-MI was demonstrated by immunostainings
[2][19]. In addition, engraftment of the CASCs after 8 weeks of acute MI has been shown to be 19%, a value higher than that previously described with other stem cells
[2][19]. It is thus very likely that CASCs are still present in the cardiac tissue 4 weeks post-transplantation. In addition, previous studies have shown that stem cells are able to secrete paracrine factors to promote survival and proliferation or have immunomodulatory effects on resident cardiomyocytes
[36][53]. The strong paracrine effects of stem cells have been well documented and are, for a part, related to the limited improvement of cardiac function seen in some studies, as those may compensate for the lack of cardiomyocyte differentiation
[37][38][54,55]. Whether the differentiation of CASCs to new cardiomyocytes and/or the paracrine factors secreted by the CASCs are the reason for the improvements seen after MI, remains to be clarified.