The CatWalk system (CW) is an automated and exceptionally reliable system for assessing gait abnormalities and motor coordination. CW is a good tool for both studying improvements in the walking of animals after suffering a peripheral nerve and spinal cord lesion and to select the best therapies and procedures after tissue destruction, given that it provides objective and quantifiable data.
1. Overview
The CatWalk system (CW) is an automated and exceptionally reliable system for assessing gait abnormalities and motor coordination. CW is a good tool for both studying improvements in the walking of animals after suffering a peripheral nerve and spinal cord lesion and to select the best therapies and procedures after tissue destruction, given that it provides objective and quantifiable data. Most studies using CW for gait analysis that were published in recent years focus on injuries inflicted in the peripheral nerve, spinal cord, and brain. CW has been used in the assessment of rodent motor function through high-resolution videos, whereby specialized software was used to measure several aspects of the animal’s gait, and the main characteristics of the automated system are presented here. CW was developed to assess footfall and gait changes, and it can calculate many parameters based on footprints and time. However, given the multitude of parameters, it is necessary to evaluate which are the most important under the employed experimental circumstances. By selecting appropriate animal models and evaluating peripheral nerve and spinal cord lesion regeneration using standardized methods, suggestions for new therapies can be provided, which represents the translation of this methodology into clinical application.
2. Motor Function
Most neurological conditions, such as central and peripheral nervous system damage, are accompanied by gait changes. It is commonly agreed upon that the details of animal limb movements during locomotion, whether in injury or during recovery from spinal cord or peripheral nerve lesions, determined using appropriate models, are of utmost importance for the assessment of such conditions. Several functional tests are used to evaluate the motor recovery after experimental nerve injury and to appraise the potential regenerative therapies. The option for certain tests depends both on the lesion model and on the costs, since specific commercial systems have higher costs.
The measurement of motor function before and after nerve injury in animal models is very important for the assessment of nerve regeneration. There are several methods for the evaluation of nerve regeneration, many of which are based on the visual observation of laboratory animals moving in an open field. Nevertheless, all these observation methods require the training of observers that can be prone to human error and subjectivity. The automated gait analysis system CatWalk XT (
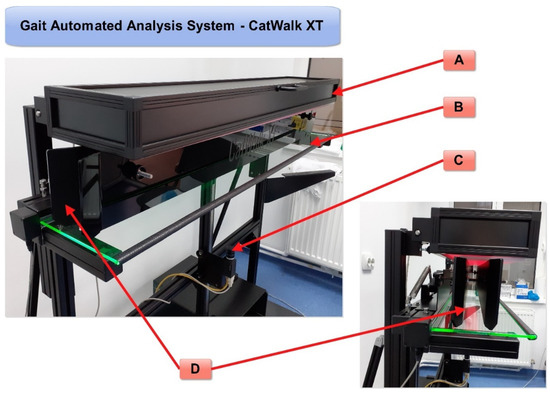
Figure 1) can overcome possible human errors of observation since specialized software is used to objectively measure several aspects of gait, and it was applied to assess rodent walk using high-resolution videos
[5][1]. Older studies have used a CW system without automatic analysis of paw parameters; therefore, we wish to focus attention on the studies that have used a CW XT with an automatic analysis system for monitoring gait and motor functions.
Figure 1. Automated gait analysis system—CatWalk XT: (A)—red LED lights in the ceiling; (B)—green LED lights along the walkway (reflected by the plantar surface of the rodent’s paw); (C)—a high-speed color camera for accurate spatial and temporal resolution; (D)—an adjustable corridor that directs the free movement of the rodents in a straight line with a glass walkway that enables CatWalk XT’s illuminated footprints technology (original photos).
Within this type of automated gait analysis, various parameters are registered and evaluated, as further presented. The experience gained using this analysis system would add information to the experimental models of neurological injuries and regeneration. In our review, we present the main features and methodology of this system, the main regeneration events that can be analyzed, and recent studies that have used this system for assessing peripheral nerve and spinal cord injuries.
4. Discussion Regarding General Outlines Using Computerized Gait Analysis System and its Effectiveness in In Vivo Experiments
In humans, minor speed changes (approximately 0.1 m/s) are predictive biomarkers of various neurological disorders, such as hemiplegic stroke
[51][2], inflammatory CNS diseases, peripheral neuropathies, vertigo syndromes, cerebrovascular CNS diseases, and idiopathic normal pressure hydrocephalus
[52][3]; therefore, systems that can objectively evaluate various gait characteristics in animal models are extremely useful.
CW is one of the very useful systems, developed in recent years, which can objectively evaluate the walk of rodents in various in vivo models of nerve recovery after injury. It is known that the experimental in vivo models for assessing the evolution of peripheral nerve and SCI before and after treatment provide valuable data regarding the methods of therapeutic approaches in humans. Translational medicine shows that the safer, more objective, more accurate, and faster the evaluation methods are, the faster the translation of data from the research bench to the patient’s bed is.
Rodents are used as the primary pre-clinical model for many diseases and the gait automated system can adequately measure a multitude of gait parameters such as static parameters (base of support, stride length, maximum contact area, relative paw position), dynamic parameters (stance, swing, and step cycle durations) and coordination parameters (regular step patterns, regularity index, phase legs, locomotor speed)
[53][4]. The animal models mimicking human neurological disorders represent the basic research avenue for both studies of disease mechanisms and of improved therapies/drugs.
Damages can occur in the central and peripheral nervous systems (e.g., stroke, trauma, tumor, degenerative neurologic disorders, or substance abuse)
[54][5]. Although rodents are not ideal models for some human diseases, they are sometimes the only preclinical models, genetically perfectible, to provide valuable preclinical experimental data.
Our purpose in this review was to analyze the results obtained in the last few years regarding the evaluation of walking parameters in spinal cord and peripheral nerve recovery after injury using CW as an objective and reliable method.
There are several methods to quantify locomotor behavior, such as electrode recordings, footprint analysis, 2D and 3D techniques for the determination of limb kinematics, visual observation of animals moving in an open field, and Gait analyzing systems
[14][6]. The latter does not replace all the others, but complement them and bring valuable evaluation data, as evidenced by the numerous studies in which it has been used. CW for rodents has proved to have many advantages, such as the speed of locomotion control and the automated data acquisition and processing. The evaluation of static and dynamic gait parameters can be performed in a variety of neurological animal models, both in central and peripheral nerve injury
[5,6,7][1][7][8]. The study of Heinzel et al. on rats with an autograft after the resection of the median nerve also showed that CW automated gait analysis is a feasible tool for assessing functional recovery in these animals. Given the complexity of quadrupedal locomotion, the advantage of this method of analysis compared to the isolated flexion of the digits becomes obvious. The CW test showed that it correlates well with other assessment methods such as grasping strength measurements and electrophysiology
[18][9]
As Kappos points out, the parameters assessed using the CW can be divided into “general parameters”, “qualitative data”, and “quantitative data”, that can be used for statistical analysis
[14][6]. Quantitative data include static and dynamic paw parameters, and coordination parameters. The authors showed that the assessment has been used mostly for nerve injury
[5][1], arthritis
[55][10], modelling pain
[50][11], but also in a few studies for neurodegenerative diseases that affect the gait
[15][12]. The system was developed to evaluate footfall and gait changes, and it can calculate 25 parameters based on footprints and 10 parameters based on time interval. It provides information for each run about paw positions, paw print sizes, and paw intensities as a function of time or video frame
[56][13].
Crowley et al. revealed that in animal models of SCI, the system can provide an unbiased quantitative assessment of the refined aspects of locomotor function and, therefore, it can detect significant differences between pre-injury and post-injury data. As stated several times in our paper, human errors and subjectivity are significantly reduced when using CW. The data obtained by taking high-resolution videos of the animals walking, analyzed with a specialized software, could be combined, creating a simple mathematical model, the Combined CatWalk Index (CCI)
[5][1]. This study showed that the CCI system produced similar results to the Basso Mouse Scale (BMS) or the CW’s Step Sequence Regularity Index, but with a significant smaller coefficient of variation. Moreover, CCI scoring showed a slightly better correlation with impact force. CCI correlated 104 CW parameters and BMS data using linear regression. All the linear regression equations were then combined into a single weighted average. The weighting factor, R
2, was used to determine parameters with strong correlation (with strong weights). The BMS method has the advantage of a single score that could be easily compared between individual mice. Nevertheless, training is required before using this method. The CW system has the advantage of a greater objectivity but choosing the right parameters for making a comparison between mice could cause problems. Thus, a combined CW index using the advantages of both systems, CCI and BMS, seemed to be a better choice. However, a significant limitation may be the linear regression model used in this work. Moreover, a correlation with spinal cord damage using histological studies was not performed. However, the authors suggested that CCI is likely to predict spinal cord tissue damage.
A recent study regarding the SCI in rodents has shown that many system’s outcome variables are highly correlated and dependent on run speed. The CW system has shown to detect significant differences between experimental groups of animals compared with the control ones. However, the summary group level data could obscure the variability between each subject of the experiment; therefore, there is a difficulty in understanding the magnitude of effect in individual rodents. In this situation, calculating reference change intervals (RCIs) is important in an experimental design for quantifying variability and providing details of individual level change. RCIs define the limits of normal variability for the values of rats locomotion scales on CW, thereby differences up to 70% from the baseline value must be considered normal
[16][14]. As the multitude of variables that CW offers to quantify the results of an experimental model of SCI, it is necessary to select the variables that define the efficacy of spinal cord intervention before the experiment begins, not restricting analysis to run speed or duration. It was suggested to use, in SCI studies, stride length, swing duration, base of support, and duty cycle as suitable measures for hind limb use. To quantify coordination, an outcome compulsory to follow, is hind limb–forelimb coupling.
Guo et al. showed that in an SCI performed by contusion or distraction, walking deficits and recovery could be affected by the type of traumatic SCI, and they used CW to perform the gait analysis of animals walking. The CW parameters for interpaw coordination (step sequence patterns and duration), paw support (base of support, print position), and front and hind paw movements (paw maximum contact area time, pressure applied to the floor, the stand time and duty cycle, paw angle, swing duration, paw stride length) were tested together with the skilled locomotor movements of animals walking across the ladder rung walking test apparatus. The two test results demonstrated their complementarity in determining the differences between the two spinal cord injury models
[29][15].
Bozkurt et al. used a rat sciatic nerve crush injury model in the CW analysis of behavioral recovery after twelve weeks
[20][16]. The group found several parameters of interest for the detection of nerve recovery, such as dynamic gait variables, coordination measures, and the intensity of paw prints, and they concluded that the system simultaneously demonstrated both static and dynamic gait parameters.
Some improvements of data presentations have also been reported for CW analysis. Hence, in order to avoid arbitrary parameter selection, heat mapping of the initial data analysis was shown to be advantageous in reporting clustered gait parameter differences
[15][12]. Moreover, a report in 2018 on the application of machine learning in CW analysis has shown that various parameters (step decomposition, the definition and extraction of meaningful features, multivariate step sequence alignment, and feature selection, among others) could be “learned”. This was the first report on the ability of machine learning to pharmacologically discriminate relevant groups based on their walking behavior
[19][17].
An evaluation of the efficacy of neurological disease treatment in in vivo models has been commonly conducted through the application of neurobehavioral tests. However, despite the attempts of numerous studies, the results cannot be applied to humans. In the opinion of Vidal et al., this discrepancy is due to poor standardization in the behavioral protocols and their execution among investigators
[57][18]. The correlation between body weight, speed, and system parameters should be calculated. For example, if an experiment lasts weeks or months, the animals’ ages and the speed of movement can be physiologically influenced not only by the protocol of the experiment but also by aging. The same can be said for body weight, a parameter known to transiently drop after injury. Many CW parameters are dependent on these two variables; therefore, instead of comparing one or two time-points using a classical
t-test or one-way and two-way ANOVA, it would be better to use models such as a linear mixed model (LMM) or linear mixed-effects (LME) model for a long-term characterization by CW
[39][19].
Another issue regarding the evaluated parameters is the standardization between laboratories. It was reported that CW could evaluate rodent motor function using specialized software that permits the diminution of inaccuracies due to human error and subjective interpretations. However, it is not always clear what data are important for the study purpose. Crowley et al. has shown that a mathematical model can be created using data collected during a mouse SCI experiment, named the Combined Catwalk Index (CCI). The CW software produces a large amount of data, with a total of approximately 104 parameters
[5][1]. Hence, the investigator must choose those parameters that are relevant and adequate to each study. The software may not be perfect but can potentially be perfected through researchers’ optimization.