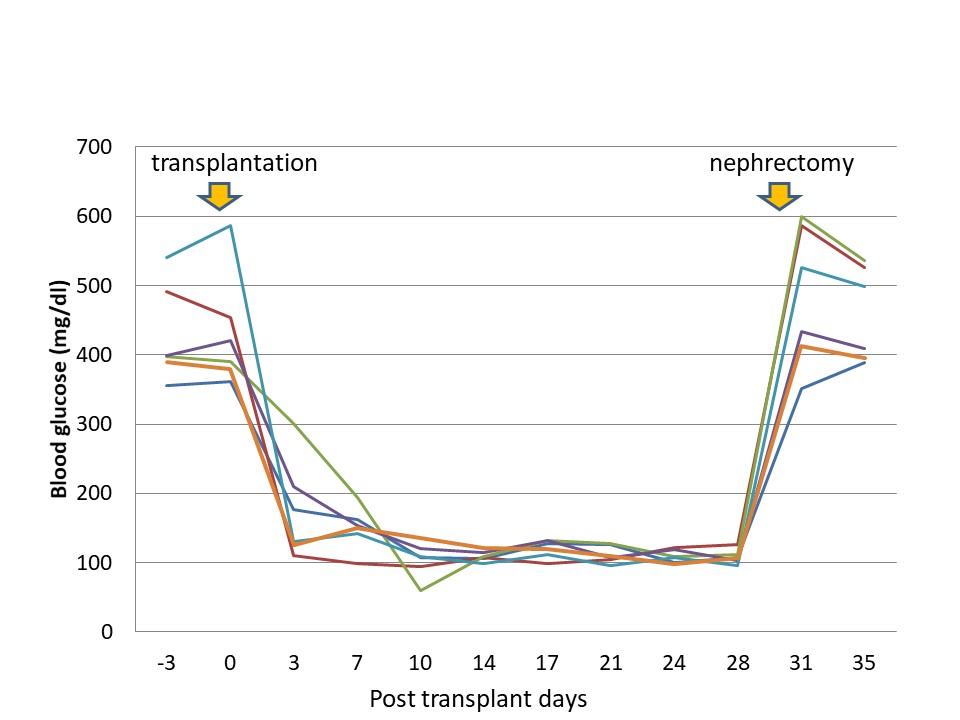
Cryopreservation of pancreatic islets enables their long-term storage and subsequent transplantation; however, post-cryopreservation, islets viability, and functions are reduced to a significant extent. Islet is composed of five cells (α cell, β cell, δ cell, ε cell, and PP cell), and blood vessels that carry the nutrition. Freezing technology of the organization has not developed a good method. This paper is studied using a fructan which has been found to effectively freeze protect a material of the cell. Islet transplantation has been established as an effective means of treating patients with type 1 diabetes. In this study, we demonstrated the effectiveness of using a fructan on the cryopreserved islets by showing valid results for diabetes. Isolated rat islets were cryopreserved using phosphate-buffered saline (PBS) supplemented with different concentrations of fructan and/or dimethyl sulfoxide (DMSO) in FBS. The survival rates of the islets were estimated at different time intervals, and insulin secretion function was tested in vitro. Furthermore, the in vivo function was tested by syngeneic transplantation into streptozotocin-induced diabetic rats, and the grafts were analyzed histologically and immunohistochemically. Fructan significantly increased islet survival; 30% fructan led to survival rates of more than 90% on day 3, which was significantly higher than those of the DMSO groups (p < 0.05). For both fructan and DMSO, the survival showed dose dependence, with the highest rates observed for 30% fructan and 10% DMSO, respectively (p < 0.05). The fructan groups showed a significantly increased insulin secretion volume in comparison to the DMSO groups (p < 0.05). Furthermore, cell clusters of pancreatic islets were well maintained in the fructan group, whereas margin collapse and vacuolation were observed in the DMSO group. Three days after transplantation of pancreatic islets preserved with 30% fructan, the blood glucose levels of diabetic rats were restored to the normal range, and removal of transplanted pancreatic islets from the kidney led to a profound increase in blood glucose levels. Together, these results show that a fructan is effective at cryopreserving rat pancreatic islets for subsequent transplantation.
- islet cryopreservation
- islet transplantation
- fructan
- serum-free freezing medium
- DMSO-free freezing medium
1. Introduction
2. Analysis on Results
2.1. Survival Rate of Islets
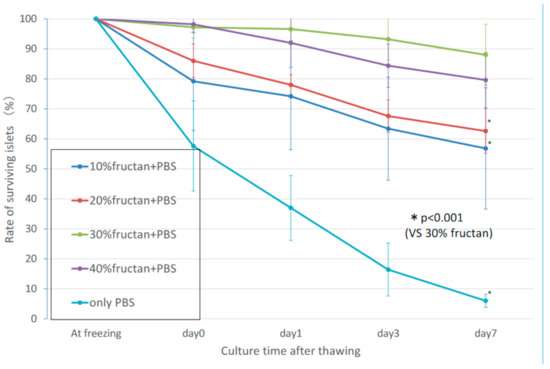
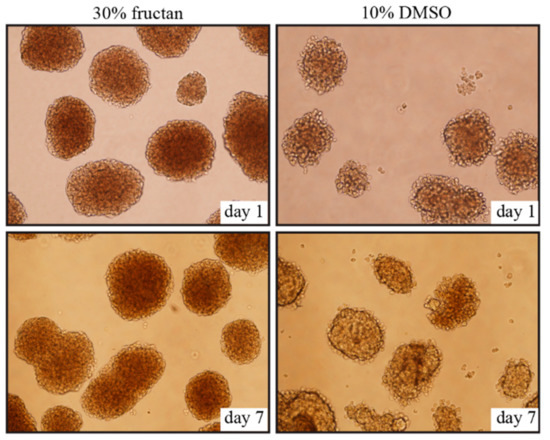
2.2. Morphology
2.3. Insulin Release Assay
| Insulin Release (n = 6) |
Low1 (ng/mL/h) |
Hi (ng/mL/h) |
Low2 (ng/mL/h) |
S.I |
|---|---|---|---|---|
| 10%fructan + PBS | 7.19 ± 1.13 | 16.54 ± 2.05 | 7.13 ± 1.31 | 2.32 ± 0.35 |
| 20%fructan + PBS | 7.03 ± 2.81 | 14.00 ± 3.38 | 7.61 ± 3.08 | 2.45 ± 1.00 |
| 30%fructan + PBS | 6.81 ± 1.41 * | 17.97 ± 5.15 * | 6.98 ± 1.35 * | 2.65 ± 0.62 |
| 40%fructan + PBS | 5.74 ± 1.90 | 13.17 ± 1.86 | 6.07 ± 2.61 | 2.55 ± 0.95 |
| 2.5%DMSO + FBS | 5.59 ± 4.90 | 8.97 ± 4.38 | 4.31 ± 2.26 | 2.32 ± 1.48 |
| 5.0%DMSO + FBS | 3.95 ± 1.09 | 8.77 ± 2.38 | 3.91 ± 1.44 | 2.43 ± 1.14 |
| 7.5%DMSO + FBS | 4.85 ± 2.18 | 9.49 ± 2.23 | 4.84 ± 2.65 | 2.25 ± 0.72 |
| 10%DMSO + FBS | 3.66 ± 2.61 | 9.93 ± 4.26 | 4.18 ± 2.78 | 3.13 ± 0.76 |
2.4. Islet Transplantation
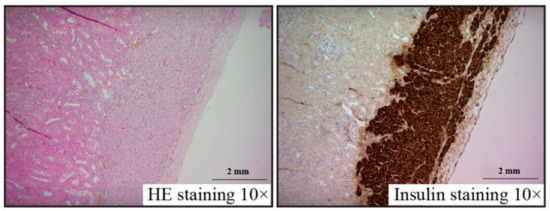
2.5. Histology
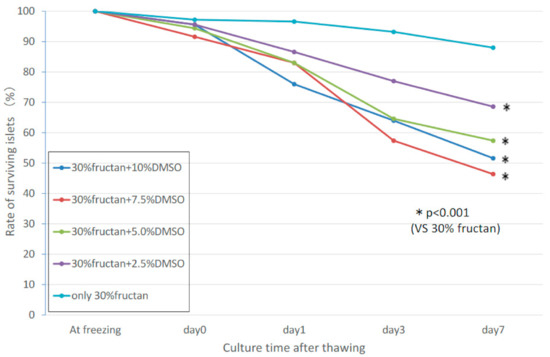
2.6. Synergistic Effects of Fructan and DMSO
| Insulin Release (n = 6) |
Low1 (ng/mL/h) |
Hi (ng/mL/h) |
Low2 (ng/mL/h) |
S.I |
|---|---|---|---|---|
| 30%fructan + PBS | 6.81 ± 1.41 * | 17.97 ± 5.15 * | 6.98 ± 1.35 * | 2.65 ± 0.62 |
| 10%DMSO + FBS | 3.66 ± 2.61 | 9.93 ± 4.26 | 4.18 ± 2.78 | 3.13 ± 0.76 |
| 30%fructan + 10%DMSO | 3.81 ± 1.48 | 11.86 ± 5.85 | 3.30 ± 2.02 | 3.11 ± 0.90 |
| 30%fructan + 7.5%DMSO | 4.91 ± 3.57 | 12.58 ± 7.58 | 4.98 ± 3.70 | 2.92 ± 1.80 |
| 30%fructan + 5.0%DMSO | 5.09 ± 1.53 | 11.59 ± 5.04 | 5.04 ± 1.78 | 2.25 ± 0.68 |
| 30%fructan + 2.5%DMSO | 4.31 ± 2.83 | 12.10 ± 6.30 | 4.80 ± 3.75 | 3.31 ± 1.12 |
