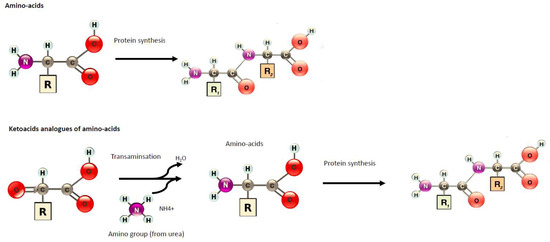
Diet is a key component of care during chronic kidney disease (CKD). In order to reduce the risk of nutritional disorders in very-low protein diets (VLDP), supplementation by nitrogen-free ketoacid analogues (KAs) have been proposed.
- chronic kidney disease
- low protein diet
- ketoacid analogues
- intestinal microbiota
- dialysis
1. Introduction
Component Name | mg/pill | ||||
|---|---|---|---|---|---|
Ca-Keto-dl-isoleucine | 67 |
Study | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Study | Models | Diet Intervention | Design of Study | Follow-Up | Diet | Results (LPD vs. VLDP/LPD + KAs) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Follow-Up | Results | Comments | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Wang et al., 2018 [7] | [8] |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Milovanova et al., 2018 [2] | [3] | 5/6 nephrectomy rats | RCT | n = 42 in LPD + KA vs. LPD n = 37 | Non-diabetic CKD 3B–4 | NPD: 22% protein | vs. | LPD: 6% protein | vs. | LPD + KAs: 5% protein plus 1% KA | LPD (0.6 g/kg of body weight/day, comprising 0.3 g of vegetable protein and 0.3 g of animal protein, phosphorus content ≤ 800 mg/day and calories: 34–35 kcal/kg/day) vs. LPD + KA: 0.6 g/kg of body weight/day | 24 weeks | 14 months | ↓ muscle atrophy | ↑ activities of mitochondrial electron transport chain complexes and mitochondrial respiration, | ↓ muscle oxidative damage | ↑ eGFR (29.1 L/min/1.73 m ↑body weight | 2 vs. 26.6) | ↓SBP | ↑BMI and muscle body mass | NO change in albumin levels | No change in lipids parameters | ↓ phosphate, FGF23, and PTH levels ↑Klotho levels and phosphate binder uses | ↑bicarbonates levels | ||||||||||||||||||||||||||||||||||||||||||||
Ca-Ketoeucine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Similar protein intake in both group | Long follow up | Liu et al., 2018 [8] | [9] | 101 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Di Iorio et al., 2018 [19] | [20] | KKAy mice, an early type 2 DN model | RCT, crossover trial | CKD stages 3B–4 | Group A1: 3 months of FD, 6 months of VLPD + KA, 3 months of FD and 6 months of MD | Group B: 3 months of FD, 6 months of MD, 3 months of FD and 6 months of VLPD + KA. | n = 30 in each group | NPD: 22% protein | vs. | LPD: 6% protein | vs. | LPD + KAs: 5% protein plus 1% KA | 12 weeks | ↓ proteinuria |
FD: proteins 1 g/kg body weight (bw)/day (animal proteins 50–70 g/day, vegetal proteins 15–20 g/day), energy 30–35 kcal/bw/day, calcium (Ca) 1.1–1.3 g/day, phosphorus (P) 1.2–1.5 g/day, sodium (Na) 6 g/day and potassium (K) 2–4 g/day. | MD: proteins 0.7–0.8 g/kg bw/day (animal proteins 30–40 g/day, vegetal proteins 40–50 g/day), energy 30–35 kcal/bw/day, Ca 1.1–1.3 g/day, P 1.2–1.5 g/day, Na 2.5–3 g/day and K 2–4 g/day. | VLPD + KA: proteins 0.3–0.5 g/kg bw/day (animal proteins 0 g/day, vegetal proteins 30–40 g/day), energy 30–35 kcal/bw/day, Ca 1.1–1.3 g/day, P 0.6–0.8 g/day, Na 6 g/day, K 2–4 g/day plus a mixture of KA | ↓ mesangial proliferation and oxidative stress |
6 months | ↑ serum albumin and body weight | No difference in creatinine and GFR | ↓ SBP | No change in creatinuria | ↓proteinuria | ↓ phosphate, FGF23, and PTH levels | ↑bicarbonates levels | ↑Hg levels | ↓protein carbamylation | Sodium intake and phosphore intake was reduce in VLDP + KA group | Ca-Ketophénylalanine | 9] | [10] | ||||||||||||||||||||||||||||||||||||
Garneata et al., 2016 [20] | 68 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
[21] | 3/4 nephrectomy rats | RCT | CKD stage 4–5, | proteinuria < 1 g/24 h | n = 207 | NPD: 18% protein | vs. | LPD: 6% protein | vs. | LPD + KAs: 5% protein plus 1% KA | 12 weeks | LPD = 0.6 g protein/kg per day | vs. | VLPD + KA = vegetarian diet, 0.3 g protein/kg per day + KA | 15 months | ↓ proteinuria | ↓ intrarenal RAS activation. | ↓ transforming growth factor-β1 in the mesangial cells | ↓ RRT initiation or a >50% reduction in the initial GFR (13% in KA+LDP vs. 42% in LPD reached the primary composite efficacy point i.e., RRT initiation or a >50% reduction in the initial GFR) | ↓CRP | ↑bicarbonates levels | ↓uric acid | ↓ phosphate, FGF23 and PTH levels and phosphate binder uses | No difference in proteinuria | No difference of death and CV events | No difference of albumin, BMI | No change in lipids parameters |
Ca-Ketovaline | 86 | |||||||||||||||||||||||||||||||||||||||
Zhang et al., 2016 [ |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Long follow up | Large effective | Only 14% of patients screened was included | Zhang et al., 2015 [10] | [11] |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Di Iorio et al., 2012 [21] | [22] | 5/6 nephrectomy rats | RCT, crossover trial | eGFR < 55 and > 20 mL/min/1.73 m 2 | Group A: VLDP + KA during the first week and LPD during the second week | Group B: LPD during the first week and a VLPD + KA during the second week. | n = 16 in each group | NPD: 11 g/kg/day protein | vs. | LPD: 3 g/kg/day protein | vs. | LPD + KAs: 3 g/kg/day protein which including 5% protein plus 1% KA | LPD = 0.6 g protein/kg per day | 24 weeks | vs. VLPD + KA = 0.3 g protein/kg per day + KA | ↑ body weight, gastrocnemius muscle mass | ↓ autophagy marker in muscle | No difference of inflammation markers | 1 week | ↓ phosphate (−12%), FGF23 (−33.5) | No change on calcium | a post hoc of this study, ↓ indoxyl sulfate [ 22] | [ ] | ↑bicarbonates levels | Short exposition | Ca-Hydroxy-dl-methionine | ||||||||||||||||||||||||||||||||||||||||||
Wang et al., 2014 [11] | [12] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Di Iorio et al., 2009 [23 | 59 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
] | [24] | 5/6 nephrectomy rats | RCT, crossover trial | eGFR < 55 and > 20 mL/min | Group A: VLDP + KA during 6 month and a LPD during 6 month | Group B: LPD during 6 month and a VLDP + KA during 6 month. | n = 16 in each group 32 patients | NPD: 22% protein | vs. | LPD: 6% protein | vs. | LPD + KAs: 5% protein plus 1% KA | LPD = 0.6 g protein/kg per day | vs. | VLPD + KA = 0.3 g protein/kg per day + KA | 24 weeks | 6 months | ↑improved protein synthesis and increased related mediators such as phosphorylated Akt in the muscle | ↓ protein degradation and proteasome activity in the muscle | ↓proteinuria and AGE | Open label | Phosphor intake was different and lower in VLDP+ KA | l-Lysine monoacetate | 105 | ||||||||||||||||||||||||||||||||||||||||||||
Gao et al., 2010 [12] | [13] |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Menon et al., 2009 [24] | [25] | 5/6 Nephrectomy rats | Post hoc study of MDRD study B | NPD: 22% protein | vs. | LPD: 6% protein | vs. | LPD + KAs: 5% protein plus 1% KA | CKD stage 4 nondiabetic | n = 255 | LPD = 0.6 g protein/kg per day | vs. | VLPD + KA = 0.3 g protein/kg per day + KA | 24 weeks | 10.2 years | ↓ proteinuria, glomerular sclerosis, and tubulointerstitial fibrosis | ↑renal function | ↑ body weight and albumin | ↓ lipid and protein oxidative products | No delay progression to kidney failure | ↑the risk of death. | Long follow up without intervention -Observance and protein intake was not monitored during the follow up | l-Threonine | |||||||||||||||||||||||||||||||||||||||||||||
Gao et al., 2011 [13] | [14] | 53 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
5/6 Nephrectomy rats | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Teplan et al., 2008 [3] | [4] |
RCT, double-blind placebo | CKD stage 4 | n = 111 | NPD: 22% protein | vs. | LPD: 6% protein | vs. | LPD + KAs: 5% protein plus 1% KA | LDP: 0.6 g protein/kg per day | vs. | LPD + KA: 0.6 g protein/kg per day + KA | 6 months | 36 months | ↑ body weight and albumin | ↓ADMA ↑ Kruppel-like factor-15, a transcription factor shown to reduce fibrosis | ↓ BMI and visceral body fat in obese patients | ↓proteinuria | l-Tryptophan | |||||||||||||||||||||||||||||||||||||||||||||||||
↓ glycated hemoglobin | ↓LDL-cholesterol | Mean BMI was > 30 kg/m2 at the inclusion | Long follow up | No difference of protein intake | Using a placebo | Maniar et al., 1992 [14] | [15] | 23 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Mircescu et al., 2007 [25] | [26] | 5/6 Nephrectomy rats | RCT | eGFR <30 mL/min/1.73 m 2, nondiabetic | n = 53 | NPD: 16% casein | vs. | LPD + EAA: 6% casein + EAA | vs. | LPD + KAs: 6% casein + KA | VLPD + KA =0.3 g/kg vegetable proteins + KA | 3 months | vs. | LPD =0.6 g/kg/d) | No difference on body weight | No difference on proteinuria vs. LDP + EAA but reduction vs. NPD | 48 weeks | ↓ creatinemia, proteinuria, glomerular sclerosis, and tubulointerstitial fibrosis vs. NPD but no difference vs. LPD + EAA | ↑survival vs. NPD but no difference vs. LPD + EAA | ↑bicarbonates levels | ↑calcium levels and ↓ phosphate | lower percentages of patients in group I required renal replacement therapy initiation (4% vs. 27%). | No change of rate of eGFR and proteinuria |
l-Histidine | 38 | |||||||||||||||||||||||||||||||||||||||||||
No change in SBP | Open label | Laouari et al., 1991 [15] | [16] |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Gennari et al., 2006 [26] | [27] | 5/6 Nephrectomy rats | Post hoc study of MDRD study | NPD: 12% casein |
l-Tyrosine | 30 |
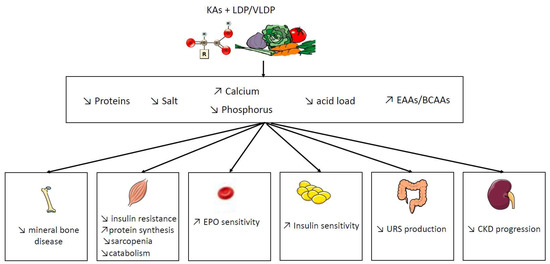
2. Potential Benefit of Ketoacid Analogues
RCT | |||||||||||||||||||||||||||||||||||
CKD stage 4–5 | |||||||||||||||||||||||||||||||||||
n | |||||||||||||||||||||||||||||||||||
= 255 | |||||||||||||||||||||||||||||||||||
vs. | |||||||||||||||||||||||||||||||||||
LPD + EAAs: 5% casein + EAA | vs. | LPD + KAs: 5% casein + KA | LPD = 0.6 g protein/kg per day | vs. | VLPD + KA = 0.3 g protein/kg per day + KA | 2,2 years | ↓Appetite and growth | No increase in BCAAs | |||||||||||||||||||||||||||
No significant effect of diet on serum total CO2 was seen | Benjelloun et al., 1993 [16] | [17] |
|||||||||||||||||||||||||||||||||
Menon et al., 2005 [27] | [28] | Rats with after a single 5 mg/kg intravenous injection of Adriamycin: a model of induces glomerular damage in glomerulonephritis. | Post oc study of MDRD study | RCT | NPD: 21% protein | vs. | LPD + KAs: 6% protein plus KA | 15 days | ↓ proteinuria | ↓ glycosaminoglycan excretion and glomerular glycosaminoglycan contents | |||||||||||||||||||||||||
CKD stage 4–5 | n = 255 | LPD = 0.6 g protein/kg per day | vs. | VLPD + KA = 0.3 g protein/kg per day + KA | 2.2 years | ↓ homocysteinemia by 24% at 1 year | Barsotti et al; 1988 [17] | [18] |
] | 5/6 Nephrectomy rats | |||||||||||||||||||||||||
Feiten et al., 2005 [28] |
RCT | n = 24 | eGFR <25 mL/min | NPD: 20.5% protein | vs. | LPD + KAs: 3.3% protein plus 7.5% KA | 3 months | VLPD + KA = 0.3 g/kg vegetable proteins + KA | vs. | LPD = 0.6 g/kg/d | ↑survival |
4 months | ↑ GFR | ↓ proteinuria and histological damage of kidney | No difference in body weight and albuminuria | ||||||||||||||||||||
[29 |
↑bicarbonates levels | No change on calcium levels | ↓ phosphate and PTH | Decrease the progression of renal decline function of rate of eGFR | No change in lipid parameters | No change in nutritional status (BMI, albumin) | Open label |
Meisinger et al., 1987 [18] | [19] |
5/6 Nephrectomy rats | LPD: 8% protein | vs. | LPD + KAs: 8% protein plus KA | 3 months | ↓ proteinuria |
Short time of follow up | |||||||||||||||||||||||||||||||||||||||
Significant reduction in dietary phosphorus (529 ± 109 to 373 ± 125 mg/day, | |||||||||||||||||||||||||||||||||||||||
p | |||||||||||||||||||||||||||||||||||||||
< 0.05) | |||||||||||||||||||||||||||||||||||||||
Prakash et al., 2004 | |||||||||||||||||||||||||||||||||||||||
[ | |||||||||||||||||||||||||||||||||||||||
29] | [30] |
RCT, double-blind placebo | eGFR:28 mL/min/1.73 m 2 | n = 34 | LPD = 0.6 g protein/kg per day + placebo | vs. | VLPD = 0.3 g protein/kg per day + KA | 9 months | preserve mGFR (−2% in LDP + KA vs. −21% in LPD) | No effect on proteinuria | No effect of BMI and albumin | Measure of GFR with 99mTc-DTPA | The placebo is problematic because protein intake was different between both groups. | ||||||||||||||||||||||||||
Teplan et al., 2003 [4] | [5] |
RCT | eGFR: 22–36 mL/min/1.73 m 2 | n = 186 | LPD 0.6 g protein/kg per day + rhuEPO + KA | vs. LPD: 0.6 g protein/kg per day + rhuEPO | vs. LPD: 0.6 g protein/kg per day | 3 years | Slower progression of CKD | ↓proteinuria | ↓LDL-cholesterol | No change in SBP | ↑albumin | ↑ plasmatic leucine levels | Role of rhuEPO unclear | Insulin clearance | |||||||||||||||||||||||
Di Iorio et al., 2003 [30] | [31] |
RCT | eGFR: < or =25 mL/min/1.73 m 2 | n = 10 in each group | LPD = 0.6 g protein/kg per day | vs. | VLPD = 0.3 g protein/kg per day + KA | 2 years | No difference on hemoglobin | ↓ EPO dose | ↓ phosphate and PTH | No change in BMI and albumin | No difference in the rate of RRT initiation (8 vs. 7) | Slower rate of GFR decline (creatinine clearance) | ↓SBP and 24 h NA excretion | ↓LDL-cholesterol | Very few populations | ||||||||||||||||||||||
Bernhard et al., 2001 [5] | [6] |
RCT | CKD stage 4–5 | n = 6 in each group | LPD = 0.6 g protein/kg per day | vs. | LPD + KA = 0.6 g protein/kg per day + KA | 3 months | No difference could be attributed to the ketoanalogs total body flux and leucine oxidation | No difference on phosphorus, calcium levels | No difference on BMI and albumin | No difference in renal function and proteinuria | No difference on bicarbonatemia | KA is metabolically safe | Short follow-up | Small effective | |||||||||||||||||||||||
Malvy et al., 1999 [31] | [32] |
RCT | eGFR<20 mL/min/1.73 m 2 | n = 50 | LPD:LPD = 0.65 g protein/kg per day + Ca+ | vs. | VLPD + KA = 0.3 g protein/kg per day + KA | 3 months or time to eGFR < 5 mL/min/1.73 m2 or RRT | No difference on GFR progression | ↑calcium levels | ↓ phosphate and PTH | No difference on lipid parameters | |||||||||||||||||||||||||||
Kopple et al., 1997 [32] | [33] |
Post hoc study of MDRD study | RCT | CKD stage 4–5 | n = 255 | LPD = 0.6 g protein/kg per day | vs. | VLPD + KA = 0.3 g protein/kg per day + KA | 2,2 years | No difference of death and first hospitalization | ↑ albumin | ↓ transferrin, body wt, percent body fat, arm muscle area, and urine creatinine excretion | No correlation between nutritional parameters and death or hospitalization | ↓ energy intake | |||||||||||||||||||||||||
Levey et al., 1996 [33] | [34] |
Post hoc study of MDRD study | RCT | CKD stage 4–5 | n = 255 | LPD = 0.6 g protein/kg per day | vs. | VLPD + KA = 0.3 g protein/kg per day + KA | 2.2 years | A 0.2 g/kg/d lower achieved total protein intake was associated with a 1.15 mL/min/yr slower mean decline in GFR (p = 0.011), which is equivalent to 29% of the mean GFR decline | Reanalyze of MDRD study by using correlations of protein intake with a rate of decline in GFR and time to renal failure | ||||||||||||||||||||||||||||
Klahr et al., 1994 Study 2 [34] | [35] |
RCT | CKD stage 4–5 | n = 255 | LPD = 0.6 g protein/kg per day | vs. | VLPD + KA = 0.3 g protein/kg per day + KA | 27 months | Marginally slower eGFR decline (−19% in LPD vs. 12% in VLDP + KA, p 0.067) | No significant interactions between blood-pressure interventions and the rate of decline in eGFR | No difference on albumin | No difference in proteinuria | -Large RCT study | -Good adherence of diet | -Measured GFR with iothalamate | ||||||||||||||||||||||||
Coggins et al. 1994 [35] | [36] |
Feasibility phase of the MDRD Study | eGFR: 8 to 56 mL/min/1.73 m 2 | n = 96 | 25 participants were excluded | LPD = 0.6 g protein/kg per day | vs. | VLPD + KA = 0.3 g protein/kg per day + KA | 6 months | No difference on lipid parameters | Pilot study | ||||||||||||||||||||||||||||
Lindenau et al. 1990 [36] | [37] |
RCT | eGFR<15 mL/min/1.73 m 2 | n = 40 | LPD = 0.6 g protein/kg per day + Ca+ vs. VLPD + KA = 0.4 g protein/kg per day + KA | 12 months | Improvement in osteo-fibrotic as well as in osteo-malacic changes | A calcium supplementation was given in LPD diet as a control for KA | |||||||||||||||||||||||||||||||
Jungers et al. 1987 [37] | [38] |
RCT | CKD stage 5 | n = 19 | LPD = 0.6 g protein/kg per day + Ca+ vs. VLPD + KA = 0.4 g protein/kg per day + KA | 12 months | No difference on biochemical or morphometric sign of de-nutrition | ↑mean renal survival duration until dialysis | Small and effective | ||||||||||||||||||||||||||||||
Hecking et al., 1982 [6] | [7] |
RCT | Mean eGFR: 10.8 mL/min/1.73 m 2 | n = 15 | LPD = 0.6 g protein/kg per day + Ca+ vs. LPD + KA = 0.6 g protein/kg per day + KA or EAA or placebo | 3 weeks per periods | ↓ phosphate | No difference on GFR and proteinuria | No difference on lipids parameters | No difference on albumin | Small and effective | versus the placebo |
FD: Free diet. P: phosphorus. MDRD: Modification of Diet in the Renal Disease Study. eGFR: estimated Glomerular Filtration Rate. RRT: renal replacement therapy. FGF23: Fibroblast Growth Factor 23. LPD: Low protein diet. VLDP: Very low protein diet. KA: Keto-analogues. RCT: randomized controlled trial. EAA: essential amino acids; PTH: parathyroid hormone.
