Peroxisome Proliferator-Activated Receptor gamma (PPARγ) is a master regulator of metabolism, adipogenesis, inflammation and cell cycle, and it has been extensively studied in the brain in relation to inflammation or neurodegeneration. Specific to viral infections is the ability to subvert signaling pathways of the host cell to ensure virus replication and spreading, as deleterious as the consequences may be for the host.
- PPAR gamma
- brain
- neural stem cells
- infection
- neuroinflammation
- HIV
- Zika
- cytomegalovirus
- neurogenesis
- microglia
1. Introduction
2. PPARγ Molecular Levers
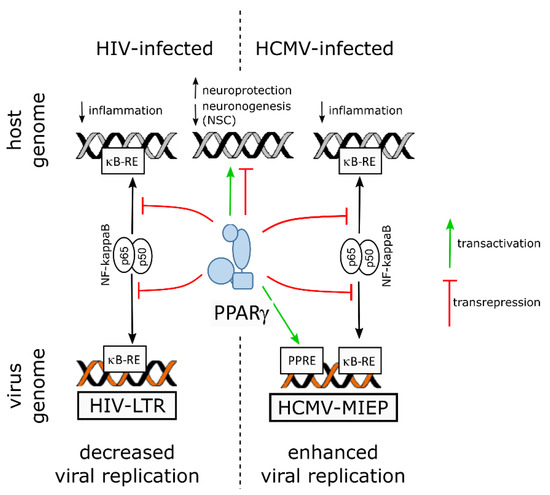
Peroxysome proliferator-activated receptors (PPARs) are members of the nuclear receptor superfamily [11][14]. As such, they are activated by lipophilic, membrane-permeant, ligands. Upon ligand binding, nuclear receptors form homo- or hetero-dimers and translocate to the nucleus to regulate gene transcription. PPAR family comprises three members, PPARα, PPARβ/δ, and PPARγ. They share a common structure containing six highly conserved functional domains: a first transcription activation function domain (AF-1), a two zinc-fingers DNA binding domain (DBD), a hinge domain, a ligand binding domain (LBD) and a second activation function domain (AF-2) that modulates binding to either co-activator or repressor factors in a ligand-dependent fashion [11][12][14,15]. The gene encoding PPARγ, namely PPARG, has a complex pattern of expression. Two alternative promoters and alternative splicing events can generate seven PPARG transcripts translated to two PPARγ isoforms: the widely expressed PPARγ1 and the adipocyte-restricted PPARγ2 [2]. Transactivation and transrepression refer to positive or negative gene transcriptional regulation by PPARγ, respectively. Transactivation requires both DNA-binding and agonist-binding whereas transrepression may require or not DNA-binding (Figure 1).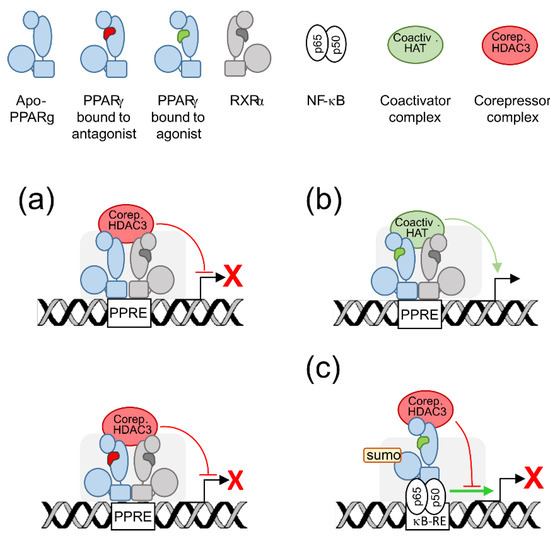
3. PPARγ Expression in the Brain
In a founder study, in situ hybridization analyses of embryonic rat brains revealed transient PPARγ mRNA expression in forebrain, midbrain and, at higher levels, hindbrain, from E13.5, to before E18.5 [3].
Few data are available on PPARγ expression in human brain due to its limited accessibility. We explored PPARγ expression by immunohistological analysis using fetal brain slices from elective abortion [16][31]. The cases were 23 to 28 gestational weeks and presented with conditions non related to brain such as (1) Digeorges syndrome (ie cardiopathy, endocrinopathy, facial dysplasia), (2) chorioamniotitis and anamnios (i.e., loss of amniotic liquid due to inflammation and premature rupture of membranes), (3) renal failure and (4) atrioventricular canal (heart dysplasia) and omphalocele (defective development of the abdominal wall). In any cases, no PPARγ was detected in any area of the brain parenchyma whereas it was detected in brain blood vessel cells. Soon after, immunofluorescence analysis of superior frontal gyrus (a part of the frontal cortex) from postmortem adult human brain has shown PPARγ expression in neurons and astrocytes but not in microglia [17][37]. Together those studies underscore that PPARγ is not evenly expressed in the brain, nor is it expressed in the same way in the fetal or adult brain, which raises the possibility that it exerts specific functions apart from its anti-inflammatory and metabolic functions.
4. PPARγ Responds to Specific Issues of the Brain Cell
4.1. Energy Supply, Oxidative Stress, and Mitochondria
PPARγ and/or PPARγ agonists were shown to exert antioxidant functions by upregulating the antioxidant enzymes haem oxygenase-1 (HO-1), catalase or copper/zinc superoxide dismutase (SOD) and downregulating the pro-oxydative enzymes inducible nitric oxide synthase (iNOS) or cyclooxygenase 2 (COX2) (reviewed in [5][7][5,7]). Rosiglitazone was also shown to prevent apoptosis related to amyloid [18][42] or tumor necrosis factor alpha (TNF-α) [19][43] in human neural stem cells by normalization of oxidative stress and mitochondrial function. Indeed, PPARγ protective role is further supported by its positive effect on mitochondria, that, beyond the cell powerhouse, are key regulators of redox balance [20][44]. A wealth of in vitro studies reviewed in [5][7][5,7] have shown that PPARγ and/or its agonists improved mitochondrial functions in human lymphocytes, adipocytes, astrocytes, neuroblastoma (SH-SY5Y) or neuronal (NT2) cell lines and hippocampal neurons, as shown by increased mitochondrial membrane potential (ΔΨm), increased mitochondrial DNA (mtDNA) copy number, modulation of mitochondrial fusion-fission events and/or expression of factors beneficial to mitochondrial biogenesis and homeostasis, namely the co-activator PGC1-α [21][45], the mitochondrial transcription factor A (TFAM) [22][46] or the nuclear factor erythroid-derived 2-like 2 Nrf2 [23][47].
4.2. Neuroinflammation
Recent findings have provided better knowledge on the protective role of PPARγ in neuroinflammation. PPARγ has been shown to mediate suppression of inflammation by the anesthetic propofol in rat astrocytes [24][57]. To note, this effect is associated with PPARγ-dependent inhibition of the Wnt/β-catenin pathway, an important pathway which enhances neuroinflammation and has a mutual positive regulation with NF-ĸB [25][58]. It has been shown that translocator protein (TSPO) inhibited microglia activation by interleukin (IL-) 4 through PPARγ activity in a primary microglia polarization model [26][59]. Rice bran extract (which is rich in PUFA) as well as pioglitazone have been reported to protect against inflammation induced by lipopolysaccharides (LPS) in a mouse model, decreasing TNF-a and COX2 levels in brain, reducing striatal plaque formation and suppressing cortical and hippocampal tissue damage, all effects requiring PPARγ activity [27][60]. Other recent studies converged to support positive, PPARγ-dependent, role against neuroinflammation of PPARγ agonists as rosiglitazone which induced IL-10 in primary rat astrocytes exposed to LPS [28][61], or pioglitazone in a rat model of chronic intermittent hypoxia [29][62].
4.3. Neurogenesis
5. PPARγ in the Infected Adult or Developing Brain
5.1. PPARγ, the Adult Brain and Human Immunodeficiency Virus 1
More recent studies have converged to highlight the beneficial role of PPARγ activation in HIV-infected brain. It has been disclosed that insulin treatment upregulated PPARγ expression in HIV-infected primary cultures of human microglia as well as in the cortex, but not in the striatum, of cats infected with feline immunodefiency virus, along with antiviral, anti-inflammatory, and neuroprotective outcomes [33][85]. Rosiglitazone was found to inhibit NF-κB as well as the release of inflammatory mediators (TNFα, IL-1β) or of iNOS and to prevent downregulation of the mouse ortholog of the glutamate transporter EAAT2 (excitatory amino acid transporter 2) caused by recombinant gp120 in primary mixed cultures of rat astrocytes and microglia or in rat after intracranial injection [34][86]. Interestingly, the same study reported a decrease in PPARγ transcript levels associated with gp120 treatment. EcoHIV is a chimeric HIV harboring gp80 from murine leukemia virus in place of gp120, thereby allowing for the infection of mouse cells and the onset of some molecular change observed in HAND [35][87]. Rosiglitazone and pioglitazone were demonstrated to reverse the increase in inflammatory mediators (TNFα, IL-1β, the chemokines CCL2, CCL3, CXCL10) and iNOS levels induced by EcoHIV in primary cultures of mouse glial cells and in mouse brains after intracranial injection [36][88]. In the same study, the two thiazolidinediones were also found to reduce in vivo EcoHIV p24 protein levels in the brain, what strongly supported an antiviral activity of the two agonists. Since then, similar results were obtained by the same group with the novel, non-thiazolidinedione, PPARγ agonist, INT131 [37][89]. PPARγ activity was however not assessed in these three reports.
Another role of PPARγ apart from neuroinflammatory modulation, has been highlighted in the context of HIV infection. Blood-brain barrier (BBB) is critical for HIV entry into the brain, and tight junction proteins are key structural and functional elements of integrity and efficiency of the BBB. In an in vitro BBB model, loss of barrier efficiency caused by HIV-infected human monocytes was shown to be reduced by overexpression of PPARγ in monocytes, in particular through repression of HIV-induced matrix metalloproteases (MMP) -2 and -9 activities [38][90].
On the virus side, NF-ĸB activity is known to be subverted to stimulate viral replication in the host cell by using the two NF-ĸB responsive elements within the promoter enhancer region of the long terminal repeat sequence (LTR) of the HIV genome [10][13]. Hence, by counteracting NF-kB through transrepression, PPARγ hampers not only inflammatory mediators release but also viral replication. Indeed, PPARγ activity was shown to suppress HIV LTR promoter activity, to decrease NF-κB occupancy of the LTR in infected cell, and, finally, to impair HIV replication in brain macrophages of an humanized mouse model of HIV encephalititis [39][96].
5.2. PPARγ, the Developing Brain and Zika Virus
Notably, PPARγ transcript levels were found to be increased in human NPCs derived from induced pluripotent stem cells (iPSC), as revealed by RNA-seq, along with productive infection, proliferation arrest and apoptosis ([40][99], and supplemental data therein). A more recent study used quantitative proteomics and transcriptomics in ZIKV-infected human NPCs and revealed, however, decreased levels of PPARγ mRNA [41][100]. The same study reported upregulation of RXRγ, of a positive regulator of PPARγ activity (Signal transducer and activator of transcription [STAT] 5 [42][101]) and of two negative regulators of PPARγ activity (FGR, a member of the Src family of tyrosine protein kinases [43][102], and the AP-1 transcription factor c-Jun [44][103]), whereas nuclear receptor coactivator 1 (NCOA1), a coactivator of both RXR and PPARγ [45][104], was found to be downregulated.
5.3. PPARγ, the Developing Brain and Human Cytomegalovirus
Infection of neural progenitor cells in the developing brain is thought to be a primary cause of the neurological sequelae due to HCMV congenital infection ([16][31] and references therein). In vitro studies showed that HCMV infection of progenitors disrupted self-renewal and polarization [46][107], apoptosis [47][108], differentiation [46][47][48][49][50][51][107,108,109,110,111,112] or migratory abilities [52][113]. Because PPARγ had been shown previously to be upregulated in human placenta cells infected by HCMV [53][114], NSCs from human embryonic stem cells were used as a model to investigate the outcomes on PPARγ activity of the infection of neural progenitors by HCMV (Figure 2) [16][31].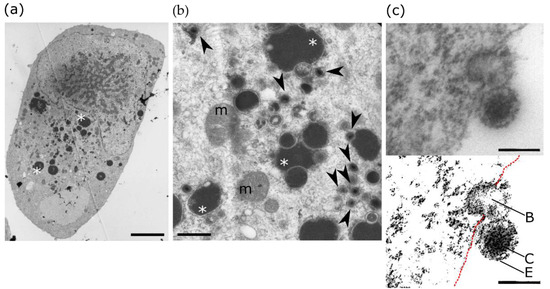
6. Conclusions