Molecular magnets, in principle, have a similar hierarchy. Starting from single-molecule magnets (SMM)and single-chain magnets (SCM), more complex structures are also possible. In general, molecular nanomagnets can be built without the aforementioned magnetic elements; however, highly interesting molecular nanomagnets can be created by adding Mn12, Fe8, Mn4, or other metallic elements.
- single-molecule magnet (SMM)
- single-chain magnet (SCM)
- molecular nanomagnet
- molecular structure
- information storage
1. Introduction
When thinking about nanomagnets from a physicist’s point of view, usually zero-dimensional (0D) or one-dimensional (1D) magnets will come to mind. Such nanomagnets may be formed, e.g., from the ferromagnets iron, nickel, cobalt, or permalloy, or from the ferrimagnets of magnetite or nickel ferrite, to name just a few [1,2,3,4,5][1][2][3][4][5]. Ferromagnetic materials contain elementary magnets for which a parallel orientation is energetically favored, while ferrimagnets can be imagined as containing two antiparallely oriented ferromagnetic sub-lattices with different magnitudes of magnetization, in this way also resulting in a net magnetization. Diverse shapes can be thought of, from square or round nanodots [6,7][6][7] to magnetic nanowires, e.g., those used in the so-called Racetrack memory [8[8][9][10],9,10], to more complicated shapes, including 3D particles [11,12,13][11][12][13]. Combining different magnetic materials, e.g., a ferromagnetic and an antiferromagnetic one, can result in additional effects as a result of the surface interactions, such as the exchange bias [14,15,16,17][14][15][16][17].
Molecular magnets, in principle, have a similar hierarchy. Starting from single-molecule magnets (SMM) [18,19,20][18][19][20] and single-chain magnets (SCM) [21,22,23][21][22][23] , more complex structures are also possible [24,25,26][24][25][26]. In general, molecular nanomagnets can be built without the aforementioned magnetic elements [27]; however, highly interesting molecular nanomagnets can be created by adding Mn 12, Fe 8, Mn 4, or other metallic elements [28,29,30][28][29][30].
2. Magnetic Interactions via a Ligand
Generally, magnetic interactions via ligands can be assumed to be similar to those found in inorganic materials. As organic molecules are usually isolating, the magnetic interactions are more localized. In the easiest approach, singly occupied molecular orbitals (i.e., unpaired electrons) inside a molecule are regarded as magnetic orbitals, with their relative orientation being responsible for ferromagnetic or antiferromagnetic coupling [37,38][31][32]. This exchange interaction works directly between unpaired electrons or via a ligand, in this case usually described as a super-exchange interaction. This interaction is depicted in more detail in Figure 1 [39][33].
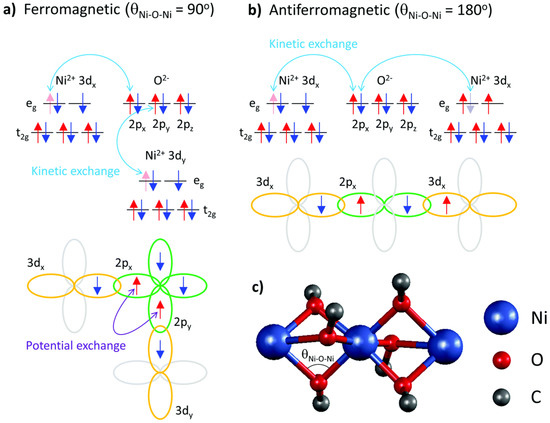
In addition, a double exchange can occur in mixed valence systems (e.g., metal ions in different oxidation states, such as Mn III and Mn IV ), describing the fast hopping of unpaired electron between the ions, i.e., delocalization of the valences. This results in a ferromagnetic coupling, as the transferred spin must be parallel to the other Mn 4+ spins because of the Pauli principle [40,41][34][35].
3. Single-Molecule Magnets
One disadvantage of the aforementioned single-molecule magnets is their very small blocking temperature, typically below the temperature of liquid helium. Ideally, magnetic properties should be reached above the temperature of liquid nitrogen , i.e., 77 K. This is one of the challenges diverse research groups have been working on during the last decades [51][36].
Much higher values were observed by Gould et al. who compared the divalent linear metallocenes Ln(Cp iPr5 ) 2 (with Ln = Tb, Dy) with the trivalent ones [Ln(Cp iPr5 ) 2][B(C 6F 5) 4], and found longer relaxation times in Tb II than in TB III and longer relaxation times in Dy III than in Dy II , and a similar behavior for Tb II and Dy III , as could be expected from the identical number of unpaired electrons in the latter. Most importantly, in Tb(Cp iPr5 ) 2 they found a high blocking temperature of 52 K [59][37].
For spin-based devices based on SMMs, controlling the spin relaxation is of the utmost importance. Sorensen et al. reported on the effect of a non-linear to pseudo-linear change in the crystal field symmetry in a dysprosium complex, leaving the residual chemistry unaltered, and found a strong reduction in the tunnel splitting at very low temperatures in the milliKelvin range [101][38]. Switching the magnetic anisotropy reversibly was enabled in lanthanide complexes as a function of external magnetic field and temperature [102,103][39][40].
Redox-active tetrathiafulvalene (TTF)-based ligands, on the other hand, allowed for designing coordination lanthanide complexes in different oxidation states, resulting in different magnetic properties for such SMMs [104][41]. An overview of such TTF-based ligands is given in [105][42].
4. Recent Trends in Molecular Nanomagnets
Besides the aforementioned topics, which are often related to basic research, some recent trends should be mentioned that are currently in the focus of research in this area, often aiming at multifunctional molecular magnets.
One of these topics is related to luminescent SMMs based on lanthanides for possible application in quantum computing, bio-labeling, or LEDs. Jia et al. recently reviewed the design strategies for such luminescent lanthanide-based SMMs, pointing out the necessary combination of a strongly UV absorbing ligand environment that efficiently populates excited states in the metal ion, with a filled-up coordination sphere of the Ln(III) ions to reduce radiation-less deactivation processes [170][43]. Yi et al. investigated the isostructural dimers [Ln(hfac) 3(PyNO)] 2 (with the lanthanides Eu, Gd, Tb, and Dy) and found luminescence for Eu, Tb, and Dy [171][44]. For Yb III , Er III , and Eu III , Jiménez et al. found luminescence in different mononuclear Ln III complexes [172][45]. Wang et al. suggested using a near-infrared emissive SMM as a highly sensitive luminescent thermometer [173][46]. A review of luminescent Schiff-base lanthanide SMMs can be found in [174][47].
Even ferroelectricity was found in SMMs [177,178][48][49] and SCMs [179,180][50][51].
As this short entry shows, multifunctional molecular nanomagnets enable combining magnetic with different optical or electrical properties, making them highly useful for diverse recent applications.
References
- Ding, J.; Adeyeye, A.O. Ni80Fe20/Ni binary nanomagnets for logic applications. Appl. Phys. Lett. 2012, 101, 103117.
- McGuigan, M.; Davenport, J.W.; Glimm, J. Computational approach to finite size and shape effects in iron nanomagnets. J. Magn. Magn. Mater. 2008, 320, 190–196.
- Sharma, N.; van Mourik, R.A.; Yin, Y.; Koopmans, B.; Parkin, S.S.P. Focused-electron-beam-induced-deposited cobalt nanopillars for nanomagnetic logic. Nanotechnology 2016, 27, 165301.
- Morais, P.C.; Lima, E.C.D.; Rabelo, D.; Reis, A.C.; Pelegrini, F. Magnetic resonance of magnetite nanoparticles dispersed in mesoporous copolymer matrix. IEEE Trans. Magn. 2000, 36, 3038–3040.
- Chatterjee, B.K.; Ghosh, C.K.; Chattopadhyay, K.K. Temperature depencence of magnetization and anisotropy in uniaxial NiFe2O4 nanomagnets: Deviations from the Callen-Callen power law. J. Appl. Phys. 2014, 116, 153904.
- Ehrmann, A.; Blachowicz, T. Vortex and double-vortex nucleation during magnetization reversal in Fe nanodots of different dimensions. J. Magn. Magn. Mater. 2019, 475, 727–733.
- Ehrmann, A.; Blachowicz, T. Systematic study of magnetization reversal in square Fe nanodots of varying dimensions in different orientations. Hyperfine Interact. 2018, 239, 8.
- Parkin, S.S.P.; Hayashi, M.; Thomas, L. Magnetic domain-wall racetrack memory. Science 2008, 320, 190–194.
- Blachowicz, T.; Ehrmann, A. Magnetization reversal in bent nanofibers of different cross-sections. J. Appl. Phys. 2018, 124, 152112.
- Kumar, D.; Jin, T.L.; Risi, S.A.; Sbiaa, R.; Lew, W.S.; Piramanayagam, S.N. Domain wall motion control for racetrack memory applications. IEEE Trans. Magn. 2019, 55, 2300708.
- Keller, L.; Al Mamoori, M.K.I.; Pieper, J.; Gspan, C.; Stockem, I.; Schröder, I.; Barth, S.; Winkler, R.; Plank, H.; Pohlit, M.; et al. Direct-write of free-form building blocks for artificial magnetic 3D lattices. Sci. Rep. 2018, 8, 6160.
- Kern, P.; Döpke, C.; Blachowicz, T.; Steblinski, P.; Ehrmann, A. Magnetization reversal in ferromagnetic Fibonacci nano-spirals. J. Magn. Magn. Mater. 2019, 484, 37–41.
- Al Mamoori, M.; Schröder, C.; Keller, L.; Huth, M.; Müller, J. First-order reversal curves (FORCs) of nano-engineered 3D Co-Fe structures. AIP Adv. 2020, 10, 015319.
- Nogués, J.; Schuller, I.K. Exchange Bias. J. Magn. Magn. Mater. 1999, 192, 203–232.
- Schneider, V.; Reinhold, A.; Kreibig, U.; Weirich, T.; Güntherodt, G.; Beschoten, B.; Tillmanns, A.; Krenn, H.; Rumpf, K.; Granitzer, P. Structural and magnetic properties of Ni/NiOxide and Co/CoOxide core/shell nanoparticles and their possible use for ferrofluids. Z. Phys. Chem. 2006, 220, 173–187.
- Tillmanns, A.; Blachowicz, T.; Fraune, M.; Güntherodt, G.; Schuller, I.K. Anomalous magnetization reversal mechanism in unbiased Fe/FeF2 investigated by means of the magneto-optic Kerr effect. J. Magn. Magn. Mater. 2009, 321, 2932–2935.
- Nogués, J.; Sort, J.; Langlais, V.; Skumryev, V.; Surinach, S.; Munoz, J.S.; Baró, M.D. Exchange bias in nanostructures. Phys. Rep. 2005, 422, 65–117.
- Christou, G.; Gatteschi, D.; Hendrickson, D.N.; Sessoli, R. Single-molecule magnets. MRS Bulletin 2000, 25, 66–71.
- Bogani, L.; Wernsdorfer, W. Molecular spintronic using single-molecule magnets. Nat. Mater. 2008, 7, 179–186.
- Sessoli, R.; Powell, A.K. Strategies towards single molecule magnets based on lanthanide ions. Coord. Chem. Rev. 2009, 253, 2328–2341.
- Coulon, C.; Miyasaka, H.; Clérac, R. Single-chain magnets: Theoretical approach and experimental systems. Struct. Bond. 2006, 122, 163–206.
- Sun, H.-L.; Wang, Z.-M.; Gao, S. Strategies towards single-chain magnets. Coord. Chem. Rev. 2010, 254, 1081–1100.
- Böhme, M.; Plass, W. How to link theory and experiment for single-chain magnets beyond the Ising model: Magnetic properties modeled from ab initio calculations of molecular fragments. Chem. Sci. 2019, 10, 9189–9202.
- Wu, B.-Y.; Yang, C.-I.; Nakano, M.; Lee, G.H. Ferromagnetic interaction and slow magnetic relaxation in a Co3 cluster-based three-dimensional framework. Dalton Trans. 2014, 43, 47–50.
- Yi, X.H.; Calvez, G.; Daiguebonne, C.; Guillou, O.; Bernot, K. Rational organization of lanthanide-based SMM dimers into three-dimensional networks. Inorg. Chem. 2015, 54, 5213–5219.
- Dutta, D.; Lefkidis, G.; Hübner, W. Role of the static correlations on the ultrafast spin dynamics of 3d molecular nano-magnets. Phys. Scr. 2020, 95, 065805.
- Takahashi, M.; Turek, P.; Nakazawa, Y.; Tamura, M.; Nozawa, K.; Shiomi, D.; Ishikawa, M.; Kinoshita, M. Discovery of a quasi-1D organic ferromagnet, p-NPNN. Phys. Rev. Lett. 1992, 69, 1290.
- Mertes, K.M.; Suzuki, Y.; Sarachik, M.P.; Myasoedov, Y.; Shtrikman, H.; Zeldov, E.; Rumberger, E.M.; Hendrickson, D.N.; Christou, G. Mn12-acetate: A prototypical single molecule magnet. Solid State Commun. 2003, 127, 131–139.
- Murrie, M. Cobalt(II) single-molecule magnets. Chem. Soc. Rev. 2010, 39, 1986–1995.
- Cirillo, G.A.; Turvani, G.; Graziano, M. A quantum computation model for molecular nanomagnets. IEEE Trans. Nanotechnol. 2019, 18, 1027–1039.
- Slater, J.C. Average energy of states of given multiplicities in atoms. Phys. Rev. 1968, 165, 655.
- Kahn, O.; Briat, B. Exchange interaction in polynuclear complexes. Part 1. Principles, model and application to the binuclear complexes of chromium. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1976, 72, 268–281.
- Domanov, O.; Weschke, E.; Saito, T.; Peterlik, H.; Pichler, T.; Eisterer, M.; Shiozawa, H. Exchange coupling in a frustrated trimetric molecular magnet reversed by a 1D nano-confinement. Nanoscale 2019, 11, 10615–10621.
- Anderson, P.W.; Hasegawa, H. Considerations on double exchange. Phys. Rev. 1955, 100, 675.
- Borras-Almenar, J.J.; Clemente-Juan, J.M.; Coronado, E.; Mirovitskii, V.Y.; Palii, A.V.; Tsukerblat, B.S. Double exchange in orbitally degenerate mixed valence clusters: Magnetic anisotropy, vibronic effects. In Vibronic Interactions: Jahn-Teller Effect in Crystals and Molecules; Kaplan, M.D., Zimmerman, G.O., Eds.; Springer: Dordrecht, The Netherlands, 2001; Volume 39, pp. 111–122.
- Shao, D.; Wang, X.Y. Development of single-molecule magnets. Chin. J. Chem. 2020, 38, 1005–1018.
- Gould, C.A.; McClain, K.R.; Yu, J.M.; Groshens, T.J.; Furche, F.; Harvey, B.G.; Long, J.R. Synthesis and Magnetism of Neutral, Linear Metallocene Complexes of Terbium(II) and Dysprosium(II). J. Am. Chem. Soc. 2019, 141, 12967–12973.
- Sorensen, M.A.; Hansen, U.B.; Perfetti, M.; Pedersen, K.S.; Bartolomé, E.; Simeoni, G.G.; Mutka, H.; Rols, S.; Jeong, M.; Zivkovic, I.; et al. Chemical tunnel-splitting-engineering in a dysprosium-based molecular nanomagnet. Nat. Commun. 2018, 9, 1292.
- Perfetti, M.; Sorensen, M.A.; Hansen, U.B.; Bamberger, H.; Lenz, S.; Hallmen, P.P.; Fennell, T.; Simeoni, G.G.; Arauzo, A.; Bartolomé, J.; et al. Magnetic anisotropy switch: Easy axis to easy plane conversion and vice versa. Adv. Funct. Mater. 2018, 28, 1801846.
- Bonde, N.A.; Petersen, J.B.; Sorensen, M.A.; Nielsen, U.G.; Fak, B.; Rols, S.; Ollivier, J.; Weihe, H.; Bendix, J.; Perfetti, M. Importance of Axial Symmetry in Elucidating Lanthanide–Transition Metal Interactions. Inorg. Chem. 2020, 59, 235–243.
- Pointillart, F.; Gonzales, J.F.; Montigaud, V.; Tesi, L.; Cherkasov, V.; Le Guennic, B.; Cador, O.; Ouahab, L.; Sessoli, R.; Kuropatov, V. Redox- and solvato-magnetic switching in a tetrathiafulvalene-based triad single-molecule magnet. Inorg. Chem. Front. 2020, 7, 2322–2334.
- Cador, O.; Le Guennic, B.; Ouahab, L.; Pointillart, F. Decorated Tetrathiafulvalene-Based Ligands: Powerful Chemical Tools for the Design of Single-Molecule Magnets. Eur. J. Inorg. Chem. 2020, 2020, 148–164.
- Jia, J.-H.; Li, Q.-W.; Chen, Y.-C.; Liu, J.-L.; Tong, M.-L. Luminescent single-molecule magnets based on lanthanides: Design strategies, recent advances and magneto-luminescent studies. Coord. Chem. Rev. 2019, 378, 365–381.
- Yi, X.H.; Bernot, K.; Pointillart, F.; Poneti, G.; Calvez, G.; Daiguebonne, C.; Guillou, O.; Sessoli, R. A luminescent and sublimable DyIII-based single-molecule magnet. Chem. A Eur. J. 2012, 18, 11379–11387.
- Jiménez, J.-R.; Díaz-Ortega, I.F.; Ruiz, E.; Aravena, D.; Pope, S.J.A.; Colacio, E.; Herrera, J.M. Lanthanide tetrazolate complexes combining single-molecule magnet and luminescence properties: The effect of the replacement of tetrazolate N3 by β-diketonate ligands on the anisotropy energy barrier. Chem. A Eur. J. 2016, 22, 14548–14559.
- Wang, J.H.; Zakrzewski, J.J.; Heczko, M.; Zychowsicz, M.; Nakagawa, K.; Nakabayashi, K.; Sieklucka, B.; Chorazy, S.; Ohkoshi, S.-I. Proton conductive luminescent thermometer based on near-infrared emissive molecular nanomagnets. J. Am. Chem. Soc. 2020, 142, 3970–3979.
- Long, J. Luminescent Schiff-base lanthanide single-molecule magnets: The association between optical and magnetic properties. Front. Chem. 2019, 7, 63.
- Guo, P.-H.; Meng, Y.; Chen, Y.-C.; Li, Q.-W.; Wang, B.-Y.; Leng, J.-D.; Bao, D.-H.; Jia, J.-H.; Tong, M.-L. A zigzag DyIII4 cluster exhibiting single-molecule magnet, ferroelectric and white-light emitting properties. J. Mater. Chem. C 2014, 2, 8858–8864.
- Li, X.-L.; Chen, C.-L.; Gao, Y.-L.; Liu, C.-M.; Feng, X.-L.; Gui, Y.-H.; Fang, S.-M. Modulation of homochiral DyIII complexes: Single-molecule magnets with ferroelectric properties. Chem. Eur. J. 2012, 18, 14632–14637.
- Bhatt, P.; Mukadam, M.D.; Meena, S.S.; Mishra, S.K.; Mittal, R.; Sastry, P.U.; Mandal, B.P.; Yusuf, S.M. Room temperature ferroelectricity in one-dimensional single chain molecular magnets [{M(Δ)M(Λ)}(ox)2(phen)2]n (M = Fe and Mn). Appl. Phys. Lett. 2017, 110, 102901.
- Bai, Y.-L.; Tao, J.; Wernsdorfer, W.; Sato, O.; Huang, R.-B.; Zheng, L.-S. Coexistence of magnetization relaxation and dielectric relaxation in a single-chain magnet. J. Am. Chem. Soc. 2006, 128, 16428–16429.
