Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yuna Kim and Version 2 by Camila Xu.
The graphene quantum dot (GQD), unlike the other graphene derivatives, is known to have distinctive optical properties showing size and edge-dependent fluorescence properties
- graphene quantum dots
- hydrogel
- organic-inorganic nanostructures
1. Introduction
Hydrogels, neither classified as completely liquid nor as solid state, are hydrophilic polymer networks which can absorb water to their porous networks. They have been of great interest to researchers in various applications such as polymer contact lenses and tissue engineering. The three-dimensional porous network structure can be modulated by controlling the ratio of monomers and crosslinking agents during polymerization. Moreover, the stimulus-responsive volume changes sensitive to temperature, pH, solvent, and electric field enables them to be applied in various biomedical applications [1][2][3][4][5][6][7][8][9][10][11][1,2,3,4,5,6,7,8,9,10,11]. In addition, the excellent optical and mechanical properties of the hydrogels can be applied to multifunctional contact lenses [12][13][14][12,13,14].
However, there have been some limitations of hydrogels for practical use. Firstly, they show poor mechanical properties with low tensile/compression strength and toughness. [5][15][16][5,15,16] Second, the conventional photoinitiators needed for hydrogel photopolymerization have an innate toxicity, limiting its usage in biological applications [17][18][17,18]. Thus, several methods have been proposed to synthesize hydrogels with enhanced mechanical properties and biocompatibility by hybridizing different nanomaterials [19][20][21][22][23][24][19,20,21,22,23,24]. Several carbon-based nanocomposites, such as carbon nanotubes, graphene oxides (GOs), or functionalized graphene sheets, have been implemented to improve the mechanical properties. However, these carbon nanomaterials lack the ability to function as photoinitiators, and therefore, additional toxic substances need to be employed to generate the radicals to trigger the polymerization [16][22][25][26][16,22,25,26].
The photoinitiator should be a photosensitive molecule with an absorption wavelength range adequate for the initiation of polymerization reaction, and the absorbed photon should possess sufficient energy to generate free radicals. In this process, the functional groups on the initiator can expedite the polymerization reaction [27][28][29][27,28,29]. The graphene quantum dot (GQD), unlike the other graphene derivatives, is known to have distinctive optical properties showing size and edge-dependent fluorescence properties [24][30][31][32][33][34][35][36][37][38][24,30,31,32,33,34,35,36,37,38]. It shows wide absorption spectra ranging from UV to visible wavelengths. Furthermore, GQDs are expected to show better biocompatibility than other inorganic semiconductor nanoparticles such as TiO2 and ZnO when they are used solely or as a composite [32][39][40][41][42][43][32,39,40,41,42,43].
Thus, we employed the dual functionality of GQDs as photoinitiators for the polymerization of polyacrylamide hydrogels and mechanical reinforcers of as-synthesized hydrogel networks (Scheme 1). They acted effectively even with sunlight as photoinitiators due to their broad absorption range, and achieved high Young’s moduli by up to 50 times. The swelling ratio was similar or slightly increased compared to the hydrogel fabricated by the conventional photoinitiator. Finally, we demonstrated a potential application for contact lenses with high transmittance (≥90%).
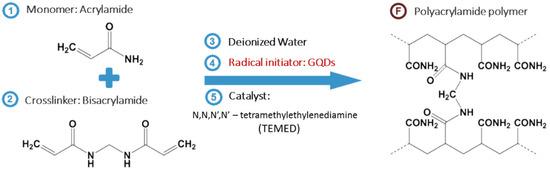
Scheme 1. Overall synthetic process of GQD-mediated hydrogel (GQDGel). The reactant (monomer and crosslinker) are dissolved in deionized water, followed by addition of initiator (GQD) and catalyst. After exposure to sunlight or UV, the final product, polyacrylamide hydrogel is synthesized.
2. Synthesis of GQDs
Graphene quantum dots (GQDs) were synthesized by following the previously reported method [32][40][41][44][32,40,41,44]. In brief, 0.9 g of carbon fiber was added into an acidic mixture of concentrated H2SO4 and HNO3 in 3:1 (v/v) ratio. The mixed solution was sonicated for two hours and stirred for 24 h at 100 and 120 °C. The mixture was cooled and diluted with deionized (DI) water. The final product was then further dialyzed (molecular weight cut-off: 1 kDa) for 3 days, followed by lyophilization. Then, 0.01 g of as-fabricated GQD was mixed with 10 mL of DI water for its usage in hydrogel.
3. Characterization of GQDs
The GQD layers were characterized using various microscopic and spectroscopic techniques. Raman analysis was performed by inVia Raman Microscope (Renishaw, Gloucestershire, UK) An absorbance spectrum was obtained by UV−Vis-NIR spectrophotometer (S-3100, Scinco, Seoul, Korea). FT-IR spectra were acquired by FT-IR spectrophotometer (Nicolet 6700, Thermo Scientific, Waltham, MA, USA). The photoluminescence characterization was performed by the fluorescence spectrometer (FP-8300, Jasco Inc., Easton, MD, USA) with Xe lamp as the source of excitation.
