Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 1 by Shu Wu.
Chicks showed heterogeneous responses to S. enteritidis infection. Enhanced intestinal barrier function and cecal microbiota structure, especially a higher abundance of Desulfovibrio_piger, may help chicks resist S. enteritidis invasion.
- Salmonella enteritidis
- heterogeneity
- cecal microbiome
- intestinal barrier
- Desulfovibrio_piger
1. Introduction
Salmonella is a major foodborne pathogen of global importance, which has led to large numbers of deaths in humans and caused economic losses in animal husbandry [1]. Among the more than 2500 identified Salmonella enterica serotypes, Salmonella enteritidis (S. enteritidis) is the most frequently spread from animals to humans globally [2]. S. enteritidis has caused occasional epidemic outbreaks around the world, such as in China [3], South Africa [4] and the United States [5]. Poultry are the primary S. enteritidis host, and the percent prevalence of S. enteritidis in chicken meat is strongly positively correlated (r = 0.804, p ≤ 0.01) with the incidence of human illnesses caused by this serotype [6]. These observations highlight the importance of studying S. enteritidis infection in poultry for reasons associated with both public health and poultry production.
Host susceptibility to pathogen infection is frequently heterogeneous [7], as demonstrated by the phenomenon of the median lethal dose (lethal dose 50 [LD50]), which describes the microbe dose that will kill only 50% of a test population [8]. Poultry infected with S. enteritidis may suffer systemic infection that can potentially lead to death, or may evolve into a long-term asymptomatic carrier-state [9]. Several studies have confirmed that heterogeneous responses to Salmonella infection can be partly explained by the genetic background and immune function status of the host [10,11][10][11]. However, numerous studies have also reported the phenomenon of heterogeneous bacterial shedding (super-shedders and low-shedders) in genetically homogeneous host populations [12[12][13],13], suggestive of the existence of additional factors that can influence the susceptibility and resistance of individuals to Salmonella colonization. Over recent years, the composition of the intestinal microbiota has been increasingly associated with heterogeneous host responses to pathogen infection [14,15,16][14][15][16].
The intestinal microbiota comprises a complex bacterial community and maintaining a mutually beneficial balance between the host and the gut microflora is very important for human health [17,18][17][18]. Intestinal dysbiosis can promote or even directly lead to a variety of conditions, including inflammatory diseases, colon cancer, and autoimmune disorders [19]. Pathogen infection is also closely related to the intestinal microbiota. Pathogen infection can lead to an imbalance in the intestinal environment, where pathogen growth is favored over that of probiotics [20,21,22][20][21][22]. Conversely, the gut microbiota can help inhibit pathogen colonization [23,24][23][24]. Although various mechanisms through which gut microbiota can protect the host against intestinal infection have been described, it remains unclear whether the heterogeneous responses of poultry to S. enteritidis infection are related to subtle changes in gut microbiota composition. In this study, we investigated the infection of S. enteritidis-susceptible and -resistant chicks from the aspects of tissue lesions, intestinal health and inflammatory response, and analyzed their cecal microbiota differences.
2. Discussion
Salmonella can be transmitted horizontally to chickens from contaminated environmental vectors and vertically from infected hens to offspring. In this study, 1-d-old female AA female chicks, with Salmonella infection excluded by cloacal swab testing, were reared and challenged under the same conditions, therefore eliminating the influence of genetic background and environment on the experimental results to the greatest extent, and ensured that all the phenotypic results obtained in this study were due to individual differences. S. enteritidis mainly colonizes the liver, spleen, and intestine of poultry after infection [41[25][26],42], leading to intestinal damage, a decline in growth performance, and even death. Growth performance, pathological changes in organs, Salmonella loads, and intestinal morphology are important indicators of the severity of S. enteritidis infection. In this study, compared with S. enteritidis-resistant chicks, the livers of S. enteritidis-susceptible chicks became swollen (Table 3) and displayed salient lesions (Figure 1b). In addition, Salmonella loads in the liver and spleen of S. enteritidis-susceptible chicks were significantly higher than those of S. enteritidis-resistant chicks (Table 31). The VCR showed an increasing trend in chicks of group R than in chicks of group S (Table 42). These results indicated that our grouping scheme, i.e., selecting chicks with differential S. enteritidis susceptibility, was appropriate, and confirmed the heterogeneous nature of the response of the birds to S. enteritidis infection.
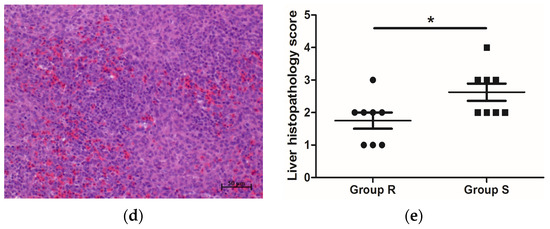
p < 0.05).

Figure 1. Histopathology of S. enteritidis-resistant and S. enteritidis-susceptible chicks. (a) Representative liver histopathology of S. enteritidis-resistant chicks (HE staining); (b) Representative liver histopathology of S. enteritidis-susceptible chicks (HE staining); (c) Representative spleen histopathology of S. enteritidis-resistant chicks (HE staining); (d) Representative spleen histopathology of S. enteritidis-susceptible chicks (HE staining). Original magnification, ×200. Black arrows indicate the lymphocytes, yellow arrows indicate the heterophilic cells, and red arrows indicate the lymphocyte nodules in liver tissue. Scale bar = 50 μm. (e) Liver histopathology score of S. enteritidis-resistant and -susceptible chicks, n = 8 per group, Result of significance test was p < 0.05 when marked *.
Table 1. Body weight, tissue index 1, and Salmonella loads of S. enteritidis-susceptible and -resistant chicks 2.
| Items | Group S 3 | Group R 3 | p-Value |
|---|---|---|---|
| BW (g) |
Table 2. Jejunum morphology of S. enteritidis-susceptible and -resistant chicks 1.
| Items | Group S 2 | Group R 2 | p-Value |
|---|---|---|---|
| 229.40 ± 7.47 | 230.88 ± 5.47 | 0.875 | |
| Liver Index (%) | 0.043 ± 0.001 a | 0.038 ± 0.001 b | 0.006 |
| 1.152 ± 0.435 | b | 0.018 | |
| Villus height (μm) | 1084.62 ± 35.20 | 1125.93 ± 90.23 | |
| Spleen Index (%) | 0.024 ± 0.002 | 0.020 ± 0.001 | 0.158 |
| Spleen Salmonella loads (log10CFU/g) | 4.784 ± 0.100 a | 2.491 ± 0.055 b | <0.001 |
1 Tissue index: Percent of tissue weight relative to body weight. 2 Results are expressed as means ± SEM, with n = 8 per group.3 Group S = selected S. enteritidis-susceptible chicks; Group R = selected S. enteritidis-resistant chicks. a,b In the same row, values with different letters are significantly different between two groups (
| 0.683 | |||||
| Crypt depth (μm) | 149.56 ± 7.48 | 131.55 ± 16.28 | 0.348 | ||
| Ratio of villus height-to-crypt depth | 7.32 ± 0.34 | 8.94 ± 0.75 | 0.090 | Liver Salmonella loads (log10CFU/g) | 2.750 ± 0.405 a |
| Muscle thickness (μm) | 117.86 ± 7.09 | 118.02 ± 14.37 | 0.992 |
1 Results are expressed as means ± SEM, with n = 8 per group. 2 Group S = selected S. enteritidis-susceptible chicks; Group R = selected S. enteritidis-resistant chicks.
The intestinal mucosal barrier serves as the first line of defense between the host and the luminal environment. Composed of epithelial cells and tight junctions, this barrier can prevent the entry of harmful substances, such as pathogens and toxins, into host tissues, organs, and circulating blood [43][27]. The intestinal epithelium is involved in the formation of the intestinal mucosal barrier by continuously secreting MUC2 to renew the intestinal mucosal layer. Impaired intestinal mucosal barrier function is a key determinant of the pathogenicity of some intestinal bacteria. Studies have shown that Salmonella infection can disrupt the intestinal barrier of broilers, and promoting the expression of tight junction proteins through L-arginine supplementation can alleviate Salmonella infection, indicating that there is a negative correlation between intestinal barrier function and the severity of Salmonella infection [40][28]. In this study, we compared the expression of genes encoding tight junction proteins and MUC2 in S. enteritidis-susceptible and S. enteritidis-resistant chicks. The results showed that the mRNA expression of occludin and MUC2 in the jejunum of S. enteritidis-resistant chicks was significantly higher than that of S. enteritidis-susceptible chicks, further supporting that a negative correlation exists between intestinal mucosal barrier function and S. enteritidis susceptibility.
Because proinflammatory cytokines are essential for initiating immune responses and eliminating pathogens in the host, we hypothesized that chicks in group R would exhibit higher levels of inflammation than those of group S, therefore explaining the greater resistance of the birds in group R to S. enteritidis infection at the same dose of S. enteritidis challenge. However, our results showed that there was no significant difference in the expression of most proinflammatory factor-related genes between the two groups. Furthermore, the gene expression of iNOS and IL6 showed the opposite trend to what would be expected, i.e., the expression of both genes was significantly higher in group S than in group R, whereas that of IL10, coding for an anti-inflammatory factor, was significantly lower. These results suggested that inflammatory cytokines may play a role in the heterogeneous responses in an unexpected way. Or the higher expression levels of proinflammatory cytokine-related genes may also be considered to be a phenotype of S. enteritidis-susceptible chicks, which is consistent with the results of the histopathological analysis of liver tissue. In addition, although iNOS is believed to help cells resist bacterial invasion through the production of a large amount of NO, which serves as an antibacterial [44][29], it is notable that the relationship between NO and Salmonella in the host may not be merely antagonistic. It has been reported that Salmonella needs NO as a nitrogen source for nitrate respiration, and a low NO concentration is indispensable for promoting Salmonella growth [45][30]. This may also explain why the invasion of S. enteritidis in birds of group S was more severe, but their expression of the iNOS gene were higher in our research.
In the chicken, the intestinal microbiota is composed of complex microbial communities that are involved in digestion and metabolism, the regulation of intestinal cells, vitamin synthesis, and the development and regulation of the host immune system [46][31]. There is also accumulating evidence indicating that the intestinal microbiota profoundly influences the pathogenicity of S. enteritidis [24]. Because the cecum is the most densely colonized microbial habitat in the chicken [47][32], we systematically compared the cecal microbial composition of chicks from the different S. enteritidis susceptibility groups. Alpha diversity refers to the richness and diversity within a microbial community in individual samples [48][33], whereas beta diversity is a comparative analysis of microbial community composition in different samples. Although no significant difference was recorded for alpha diversity, significant differences in beta diversity were observed between the cecal samples of the two groups, which agreed with previous results showing that Salmonella infection can lead to changes in cecal microbiota [21].
The cecal microbial composition of the two groups at both the phylum and genus levels was analyzed using the Wilcoxon test. The results showed that at the phylum level, the relative abundance of Acidobacteria, Campilobacterota, and Fusobacteriota were enriched in group S. The same results were obtained using LEfSe. At the genus level, 18 genera were identified as significantly differential microorganisms by the Wilcoxon test. Among them, Fusobacterium, Helicobacter, and Butycicoccus were identified as marker microorganisms in group S using LEfSe. As we know, Fusobacterium has been associated with gastric ulcers in pigs [49][34] and colon carcinoma in humans [50[35][36],51], and may represent a kind of new opportunistic pathogens of chickens worthy of further investigation [52][37]. In addition, in the species level, Helicobacter_pullorum has also been identified as a marker microorganism of group S, which is member of Campilobacterota and a well-known zoonotic pathogen [53][38]. These results revealed that chicks showing higher S. enteritidis resistance has lower abundance of pathogenic bacteria in their cecal.
Furthermore, we identified a marker microorganism, Desulfovibrio_piger, which was enriched in chicks of group R. Desulfovibrio_piger, belonging to Desulfovibrio spp., is a kind of sulfate reducing bacteria, which can functional reducing sulfate to hydrogen sulfide (H2S) and plays an important role in intestinal hydrogen and sulfur metabolism. Although H2S has been found to have dichotomous effects (stimulatory and inhibitory) on several gastrointestinal processes, it seems to be hazardous at high concentrations but favorable at low concentrations, and the overarching effect of H2S appears to be beneficial. For example, H2S can attenuate DSS-induced colitis, lessen the shortening of the colon lengths and colonic pathological damages, showing an overall protective effect in colitis via its anti-inflammatory properties [54][39]. In addition, ATB-429, an H2S releasing derivative of mesalamine, exhibits a marked increase in anti-inflammatory activity and potency in a murine model of colitis, as compared to mesalamine, seems promising in the treatment of inflammatory bowel disease [55][40]. Our results were consistent with these above reports, as our chicks in group R showed higher abundance of Desulfovibrio_piger and lower inflammation response at the same time. However, whether Desulfovibrio_piger can really help chicks to resist the infection of S. enteritidis by producing H2S still need to be verified.
3. Conclusions
In conclusion, our results confirmed that chicks showed heterogeneous responses to S. enteritidis infection, including different degrees of Salmonella loads in tissues, different tissue lesion severity, and distinct inflammatory responses. Our findings suggested that enhanced intestinal barrier function and cecal microbiota structure, especially a higher abundance of Desulfovibrio_piger, may help chicks resist S. enteritidis invasion.
References
- Shi, S.; Wu, S.; Shen, Y.; Zhang, S.; Xiao, Y.; He, X.; Gong, J.; Farnell, Y.; Tang, Y.; Huang, Y.; et al. Iron oxide nanozyme suppresses intracellular Salmonella enteritidis growth and alleviates infection in vivo. Theranostics 2018, 8, 6149–6162.
- WHO. Salmonella (Non-Typhoidal). 2018. Available online: http://www.who.int/mediacentre/factsheets/fs139/en/ (accessed on 20 February 2018).
- Jiang, M.; Zhu, F.; Yang, C.; Deng, Y.; Kwan, P.S.L.; Li, Y.; Lin, Y.; Qiu, Y.; Shi, X.; Chen, H.; et al. Whole-genome analysis of Salmonella enterica serovar enteritidis isolates in outbreak linked to online food delivery, Shenzhen, China, 2018. Emerg. Infect. Dis. 2020, 26, 789–792.
- Smith, A.M.; Tau, N.P.; Ngomane, H.M.; Sekwadi, P.; Ramalwa, N.; Moodley, K.; Govind, C.; Khan, S.; Archary, M.; Thomas, J. Whole-genome sequencing to investigate two concurrent outbreaks of Salmonella enteritidis in South Africa, 2018. J. Med. Microbiol. 2020, 69, 1303–1307.
- Sher, A.A.; Mustafa, B.E.; Grady, S.C.; Gardiner, J.C.; Saeed, A.M. Outbreaks of foodborne Salmonella enteritidis in the United States between 1990 and 2015: An analysis of epidemiological and spatial-temporal trends. Int. J. Infect. Dis. 2021, 105, 54–61.
- Shah, D.H.; Paul, N.C.; Sischo, W.C.; Crespo, R.; Guard, J. Population dynamics and antimicrobial resistance of the most prevalent poultry-associated Salmonella serotypes. Poult. Sci. 2017, 96, 687–702.
- Steed, A.L.; Christophi, G.P.; Kaiko, G.E.; Sun, L.; Goodwin, V.M.; Jain, U.; Esaulova, E.; Artyomov, M.N.; Morales, D.J.; Holtzman, M.J.; et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science 2017, 357, 498–502.
- Sanchez, K.K.; Chen, G.Y.; Schieber, A.M.P.; Redford, S.E.; Shokhirev, M.N.; Leblanc, M.; Lee, Y.M.; Ayres, J.S. Cooperative metabolic adaptations in the host can favor asymptomatic infection and select for attenuated virulence in an enteric pathogen. Cell 2018, 175, 146–158.
- Velge, P.; Cloeckaert, A.; Barrow, P. Emergence of Salmonella epidemics: The problems related to Salmonella enterica serotype enteritidis and multiple antibiotic resistance in other major serotypes. Vet. Res. 2005, 36, 267–288.
- Calenge, F.; Kaiser, P.; Vignal, A.; Beaumont, C. Genetic control of resistance to salmonellosis and to Salmonella carrier-state in fowl: A review. Genet. Sel. Evol. 2010, 42, 11.
- Chaussé, A.M.; Grépinet, O.; Bottreau, E.; Vern, L.Y.; Menanteau, P.; Trotereau, J.; Robert, V.; Wu, Z.; Kerboeuf, D.; Beaumont, C.; et al. Expression of Toll-like receptor 4 and downstream effectors in selected cecal cell subpopulations of chicks resistant or susceptible to Salmonella carrier state. Infect. Immun. 2011, 79, 3445–3454.
- Lawley, T.D.; Bouley, D.M.; Hoy, Y.E.; Gerke, C.; Relman, D.A.; Monack, D.M. Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infect. Immun. 2008, 76, 403–416.
- Menanteau, P.; Kempf, F.; Trotereau, J.; Virlogeux-Payant, I.; Gitton, E.; Dalifard, J.; Gabriel, I.; Rychlik, I.; Velge, P. Role of systemic infection, cross contaminations and super-shedders in Salmonella carrier state in chicken. Environ. Microbiol. 2018, 20, 3246–3260.
- Brown, R.L.; Clarke, T.B. The regulation of host defences to infection by the microbiota. Immunology 2017, 150, 1–6.
- Flemer, B.; Warren, R.D.; Barrett, M.P.; Cisek, K.; Das, A.; Jeffery, I.B.; Hurley, E.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. The oral microbiota in colorectal cancer is distinctive and predictive. Gut 2018, 67, 1454–1463.
- Masetti, G.; Moshkelgosha, S.; Köhling, H.L.; Covelli, D.; Banga, J.P.; Berchner-Pfannschmidt, U.; Horstmann, M.; Diaz-Cano, S.; Goertz, G.E.; Plummer, S.; et al. Gut microbiota in experimental murine model of Graves’ orbitopathy established in different environments may modulate clinical presentation of disease. Microbiome 2018, 6, 97.
- Jie, Z.; Xia, H.; Zhong, S.L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845.
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156.
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270.
- Juricova, H.; Videnska, P.; Lukac, M.; Faldynova, M.; Babak, V.; Havlickova, H.; Sisak, F.; Rychlik, I. Influence of Salmonella enterica serovar enteritidis infection on the development of the cecum microbiota in newly hatched chicks. Appl. Environ. Microbiol. 2013, 79, 745–747.
- Borewicz, K.A.; Kim, H.B.; Singer, R.S.; Gebhart, C.J.; Sreevatsan, S.; Johnson, T.; Isaacson, R.E. Changes in the porcine intestinal microbiome in response to infection with Salmonella enterica and Lawsonia intracellularis. PLoS ONE 2015, 10, e0139106.
- Pollock, J.; Hutchings, M.R.; Hutchings, K.E.K.; Gally, D.L.; Houdijk, J.G.M. Changes in the ileal, but not fecal, microbiome in response to increased dietary protein level and enterotoxigenic Escherichia coli exposure in pigs. Appl. Environ. Microbiol. 2019, 85, e01252-19.
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89.
- Litvak, Y.; Mon, K.K.Z.; Nguyen, H.; Chanthavixay, G.; Liou, M.; Velazquez, E.M.; Kutter, L.; Alcantara, M.A.; Byndloss, M.X.; Tiffany, C.R.; et al. Commensal Enterobacteriaceae protect against Salmonella colonization through oxygen competition. Cell Host. Microbe 2019, 25, 128–139.
- Shah, D.H.; Zhou, X.; Kim, H.Y.; Call, D.R.; Guard, J. Transposon mutagenesis of Salmonella enterica serovar enteritidis identifies genes that contribute to invasiveness in human and chicken cells and survival in egg albumen. Infect. Immun. 2012, 80, 4203–4215.
- Wang, C.L.; Fan, Y.C.; Wang, C.; Tsai, H.J.; Chou, C.H. The impact of Salmonella enteritidis on lipid accumulation in chicken hepatocytes. Avian. Pathol. 2016, 45, 450–457.
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol. 2016, 7, e196.
- Zhang, B.; Li, G.; Shahid, M.S.; Gan, L.; Fan, H.; Lv, Z.; Yan, S.; Guo, Y. Dietary l-arginine supplementation ameliorates inflammatory response and alters gut microbiota composition in broiler chickens infected with Salmonella enterica serovar Typhimurium. Poult. Sci. 2020, 99, 1862–1874.
- Nathan, C.; Shiloh, M.U. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 2000, 97, 8841–8848.
- Fu, Y.; Galán, J.E. A Salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature 1999, 401, 293–297.
- Khan, S.; Chousalkar, K.K. Salmonella Typhimurium infection disrupts but continuous feeding of Bacillus based probiotic restores gut microbiota in infected hens. J. Anim. Sci. Biotechnol. 2020, 11, 29.
- Pourabedin, M.; Zhao, X. Prebiotics and gut microbiota in chickens. FEMS. Microbiol. Lett. 2015, 362, fnv122.
- Li, B.; Zhang, X.; Guo, F.; Wu, W.; Zhang, T. Characterization of tetracycline resistant bacterial community in saline activated sludge using batch stress incubation with high-throughput sequencing analysis. Water. Res. 2013, 47, 4207–4216.
- De Witte, C.; Demeyere, K.; De Bruyckere, S.; Taminiau, B.; Daube, G.; Ducatelle, R.; Meyer, E.; Haesebrouck, F. Characterization of the non-glandular gastric region microbiota in Helicobacter suis-infected versus non-infected pigs identifies a potential role for Fusobacterium gastrosuis in gastric ulceration. Vet. Res. 2019, 50, 39.
- Mármol, I.; Sánchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M.J. Colorectal carcinoma: A general overview and future perspectives in colorectal cancer. Int. J. Mol. Sci. 2017, 18, 197.
- Rubinstein, M.R.; Baik, J.E.; Lagana, S.M.; Han, R.P.; Raab, W.J.; Sahoo, D.; Dalerba, P.; Wang, T.C.; Han, Y.W. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/β-catenin modulator Annexin A1. EMBO Rep. 2019, 20, e47638.
- Kollarcikova, M.; Kubasova, T.; Karasova, D.; Crhanova, M.; Cejkova, D.; Sisak, F.; Rychlik, I. Use of 16S rRNA gene sequencing for prediction of new opportunistic pathogens in chicken ileal and cecal microbiota. Poult. Sci. 2019, 98, 2347–2353.
- Javed, S.; Gul, F.; Javed, K.; Bokhari, H. Helicobacter pullorum: An emerging zoonotic pathogen. Front. Microbiol. 2017, 8, 604.
- Qin, M.; Long, F.; Wu, W.; Yang, D.; Huang, M.; Xiao, C.; Chen, X.; Liu, X.; Zhu, Y.Z. Hydrogen sulfide protects against DSS-induced colitis by inhibiting NLRP3 inflammasome. Free Radic. Biol. Med. 2019, 137, 99–109.
- Fiorucci, S.; Orlandi, S.; Mencarelli, A.; Caliendo, G.; Santagada, V.; Distrutti, E.; Santucci, L.; Cirino, G.; Wallace, J.L. Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br. J. Pharmacol. 2007, 150, 996–1002.
More
