You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Marta Mosquera.
This entry describes the most common types of bioplastics and biopolymers, and focuses specifically on the polymerization of terpenes and terpenoids, which represent a source of promising monomers to create bio-based polymers and copolymers.
- terpenes
- terpenoids
- bio-based polymers
- biopolymers
- catalyst
- REX
- myrcene
- limonene oxide
- biorefinery
1. Introduction
The rapid growth of the world’s population and the current model of the linear economy has led us not only to a rapid depletion of natural resources but also to the overexploitation of the environment and to an exponential increase of greenhouse gases emissions with very important consequences on climate change. These negative effects have forced the main economic powers to change towards a circular economy, a model based on maintaining the product’s added value as long as possible so that resources remain in the production chain even when a product has reached its end-of-life. As such, in addition to the final product, circular economy takes into account, the raw materials, the energy use, the production processes, the by-products generated in the synthesis, together with the industrial and consumer residues, packaging, transport, reuse, and recycling.
On the other hand, a bioeconomy involves the use of renewable biological resources to produce food, feed, materials, energy, and services, and it includes the primary production sectors that produce these biological resources and the manufacturing industrial sectors that use them. The establishment of a circular economy based on the bioeconomy offers great possibilities to generate and maintain economic growth and jobs in rural areas, reduce dependence on fossil fuels and improve the economic, social, and environmental sustainability of primary production and processing industries [1]. In this context, the biorefineries have emerged as an industry focused on the direct conversion of biomass onto bioenergy and high value-added products.
Biomass is defined as the biological material coming from plants, animals, microorganisms, and derived materials, including organic wastes, and they must not compete with current resources for food or feed [2,3][2][3]. Among biological resources, the most abundant feedstock is the lignocellulosic biomass, corresponding to structural foliage material. It is composed mainly of cellulose, hemicellulose, and lignin, where cellulose forms rigid fibers, embedded in a matrix of lignin and hemicellulose. Cellulose is an unbranched homopolymer of hundreds to thousands of glucose residues joined by β-1,4 linkages, whereas hemicellulose is a branched heteropolymer composed of 80–200 residues of pentoses (mainly D-xylose and L-arabinose), hexoses (mainly D-galactose, D-glucose, and D-mannose), hexuronic acids (D-galacturonic acid, D-glucuronic acid, and 4-O-methyl-D-glucuronic acid) and, to a lesser degree, 6-deoxy-hexoses (L-rhamnose, and L-fucose) and different neutral O-methylated sugars. Lignin, meanwhile, is a branched non-carbohydrate polymer of coniferyl, sinapyl, and p-coumaryl alcohols, although many plants contain significant levels of other unusual components [4]. The ratio of the different polymers depends on the type of the plant: woody feedstocks are richer in lignin and cellulose, whereas herbaceous feedstocks are richer in hemicellulose and extractives [5], nonstructural components which can be extracted by water and organic solvents (such as simple sugars, starches, proteins, fats, fatty acids, sterols, essential oils, phenolics, terpenes, flavonoids, pectins, waxes, gums, resins, and other organic compounds) [6]. Therefore, lignocellulosic feedstocks are rich in carbohydrates (higher than 60%), which can be converted by microorganisms in a fermentative process into high value-added products. Complete conversion comprises three main steps: a pretreatment to break the structure of the biomass making both cellulose and hemicellulose accessible for lytic enzymes, the release of sugars by enzymatic hydrolysis, and conversion by fermentation of monomeric sugars into energy or valuable chemicals [7]. In recent years, the production of biopolymers from renewable resources like lignocellulosic feedstock has been increasing due to environmental, political, and economic concerns about conventional plastics utilization. A wide range of biopolymers with several applications possibilities can be produced from lignocellulosic biomass, allowing the replacement of many conventional plastics [8]. In addition, other kinds of precursors such as terpenes, vegetable oils, and carbohydrates that can be obtained from biomass are very good candidates to be used as monomers.
2. Biopolymers and Bioplastics
Most petroleum-based plastics have good mechanical properties such as toughness, flexibility, and resistance, however, when disposed they persist in the environment for a long time [9]. Thus, the growing interest in the use of bioplastics is related to the benefits they offer from an environmental point of view. They reduce the dependency on fossil-based raw materials and can reduce environmental problems.
Where biomass is used as the renewable raw material, to obtain polymers, these types of polymers are referred to as bioderived [10]. However, not all are biodegradable. Bioplastics are a family of plastics that can be divided into two categories, biodegradable and non-biodegradable [11]. At this point, we have considered that some petrochemical polymers are biodegradable and that not all bioderived polymers will biodegrade.
2.1. General Considerations of Biopolymers and Bioplastics
According to the recommendation for terminology and characterization of biopolymers and bioplastics, the term bioplastic is applied to different types of materials: (i) bio-based plastics, referred to the source of their raw materials, such as Bio-PE, Bio-PU, Bio-PA, (ii) biodegradable plastics, referred to their functionality, such as: cellulose, starch and polylactic acid (PLA), and (iii) bio-compatible plastics if the material is compatible with human and animal bodies [12]. Based on the source and biodegradation, polymers can be classified as it is shown in Figure 1.
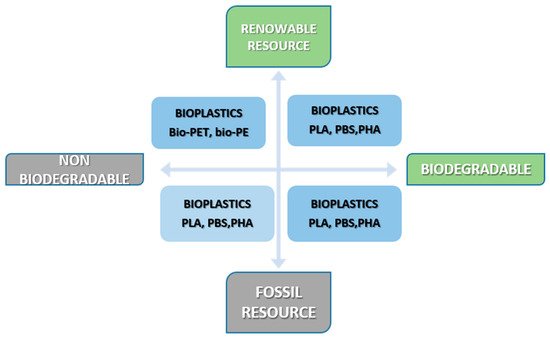
The sources that can be used for bioplastic production are plant-based raw materials, natural polymers (carbohydrates, proteins, etc.), and other small molecules (sugar, disaccharides, and fatty acids) [14].
Bio-based plastics can be natural or synthetic. The natural bioplastics can be synthesized by live organisms (plants, animals, microorganisms) through direct exploitation (extraction and purification) or indirect by functionalization of a natural polymer by chemical processes (polysaccharides, proteins, and polyhydroxyalkanoates). On the other hand, synthetic bio-based polymers are obtained from monomers derived from renewable sources through chemical conversion (such as polylactic acid PLA, bio-PE obtained from bioethanol, and polyamide 11 obtained from castor oil) [15].
Biodegradable bioplastics can be degraded by microorganisms through two different pathways—aerobic and anaerobic degradation—depending on the presence or absence of oxygen, respectively, and it is highly dependent on the properties of the plastic nature [16].
Environmental considerations are the main advantages to use this type of polymers, however, other factors such as the low production capacity compared with the conventional materials, reduces the potential substitution and increases the costs of bioplastics—see Figure 2 for a summary of advantages and aspects to be improved related with bioplastics [17]. Therefore, more efforts must be made to develop sustainable and circular alternatives for preserving resources for future generations, focusing on biodegradable and bio-renewable materials [11].

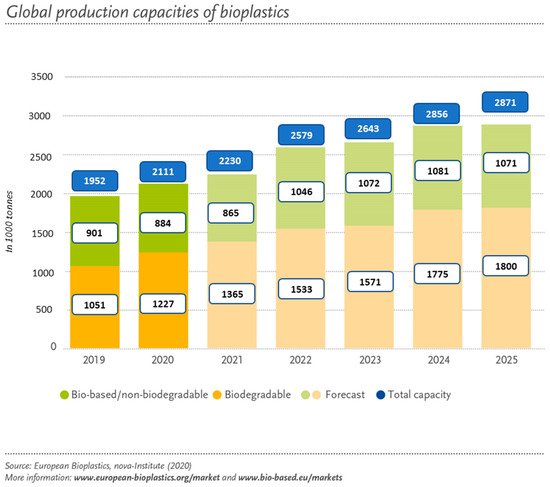
Regarding their market, it is expected to grow bigger in the future due to the social concerns and economic problems which have triggered the development of environmentally friendly materials [18]. Although amongst the plastic produced annually, bio-based plastics represent only 1% in Europe, the demand for bio-based materials is expected to grow from 2.11 million tonnes in 2020 to approximately 2.87 million tonnes in 2025 according to European Bioplastics, see Figure 3 [19].
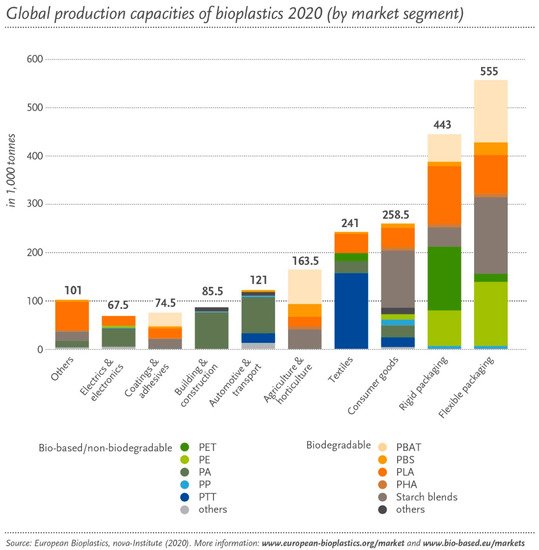
The main applications for bioplastics are packaging, food services, agriculture, consumer electronics, automotive, consumer goods, and household appliances. Packaging was the sector where the highest volume of bioplastics was used in 2020 (47%), followed by consumer goods (12%) and textiles (11%), and covering coatings (4%) (see Figure 4).
2.2. Types and Properties of Bioplastics
Generally, bio-based polymers are classified into three classes. The first class is naturally derived from biomass, such as cellulose, cellulose acetate, starches, chitin, or chemically modified ones such as modified starch, etc. The second class is bio-synthesized by using microorganisms and plants like poly(hydroxy alkanoates (PHAs). Regarding the third class are synthetic polymers such as polylactide (PLA), poly(butylene succinate) (PBS), bio-polyolefins, and bio-poly(ethylene terephthalic acid) (bio-PET). This third class is the most promising because the introduction of the bio-monomers in the existing production system for oils monomers could be possible. Monomers of this third class are naturally produced or are the result of combining chemical and biochemical processes [20].
Many biopolymers are already being produced commercially on large scale, such as cellulose, starch, collagen, casein, soy protein, and polyester [21]. Other biopolymers available in the market are polyesters like polylactic acid (PLA), polyhydroxyalkanoates (PHA), or bio-polyethylene terephthalate (bio-PET), polyvinyl alcohol (PVA) and bio-polyethylene (bio-PE), bio-polyvinyl chloride (bio-PVC), bio-polyurethane (bio-PUR), and bio-polyamides (bio-PA). Bioplastics sometimes show inferior properties compared to their fossil-based polymer counterparts. Thus, in order to improve their properties biopolymers can be blended. This approach is fast and cheap and enables the rise of competitiveness for this type of polymer. Some properties such as processability, flexibility, and glass transition temperature (Tg) are improved in PLA, PCL, PBS, PHA if miscibility and compatibility are addressed [22]. Although this approach is a good possibility, in recent years the improvements and increase in the number of commercial grades found in the market have reduced the need to deal with this blending process.
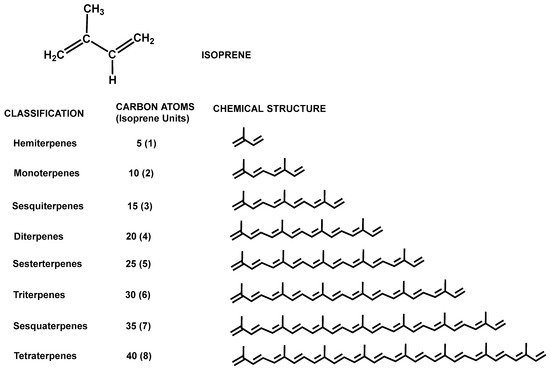 The classification of terpenes and terpenoids follows the so-called isoprene rule (C5 rule), which is based on the number and organization of carbon atoms in the molecule, thus, grouping these molecules according to the number of isoprene units linked in a head-to-tail manner and constituting hemiterpenes (1 isoprene unit), monoterpenes (2 isoprene units), sesquiterpenes (3 isoprene units), diterpenes (4 isoprene units), etc. (Figure 5). Although the head-to-tail configuration is the most common, non-head-to-tail condensation of isoprene units also exists, such as the head-to-middle fusions occurring in irregular monoterpenoids [36][30]. In addition, isoprene units can conform to chains that can be arranged to form rings.
Hemiterpenes are formed by one isoprene unit. The main hemiterpene is isoprene (methylbuta-1,3-dien, C5H8), a volatile compound commonly found in the atmosphere that results from plant metabolism and is emitted by leaves as a natural byproduct [37,38][31][32]. Isoprene is produced in large amounts by phytoplankton in the oceans, and animals and bacteria can also emit it. Isoprene production in plants appears to be related to the protection of photosynthesis and other processes under stress conditions [39][33]. Plants can synthesize other hemiterpenes, such as several acids (tiglic, angelic, isovaleric, and senecioic) and isoamyl alcohol [40[34][35],41], which are responsible for flavors and aromas.
Monoterpenes comprise two isoprene units (10 carbon atoms; C10H16). These compounds are present in plant oils and are characterized by their strong aroma and odor (used as components of perfumes and cosmetics), and for their biological activity [42,43][36][37]. Monoterpenes can be simple unsaturated hydrocarbons or constitute alcohols, aldehydes, and ketones. They are classified as acyclic or aliphatic (e.g., myrcene, citral, geraniol, lavandulol, and linalool), monocyclic (e.g., α-terpineol, limonene, thymol, menthol, carvone, eucalyptol, and perillaldehyde), and bicyclic, the latter containing a six-member ring and a second ring that can have either three, four, or five members (6 + 3: thujone and Δ3-carene; 6 + 4: α- and β-pinene; 6 + 5: borneol and camphor) [41,44][35][38]. Biosynthesis of α-pinene has been achieved in a genetically engineered Yarrowia lipolytica using low-cost renewable feedstocks [45][39].
A large subtype of monoterpenes is formed by iridoids, which contain a six-membered ring with an oxygen atom fused to a cyclopentane ring (iridane skeleton). They are usually classified as glycosides, since they occur in plants and are often combined with sugars, e.g., aucubin, harpagoside [46][40]. Iridoids are present in medicinal plants and used as sedatives, antipyretics, remedies for wounds, skin disorders, and hypotensives [47][41].
Sesquiterpenes are the most diverse group of terpenoids [48][42]. They consist of three isoprene units (C15H24) and can be naturally found in many latex-producing plants. These compounds are present in linear or acyclic (e.g., farnesol, β-nerolidol), monocyclic (e.g., β-bisabolene, α-zingiberene, α-humulene), bicyclic (e.g., β-santalol, β-caryophyllene, artemisinin), and tricyclic (e.g., khushimol, thujopsene) forms. Sesquiterpenes can also give rise to γ-lactone ring structures (sesquiterpene lactones), thus constituting germacranolides (e.g., costunolide, parthenolide), eudesmanolides (e.g., santonin, alantolactone), and guaianolides (e.g., artabsin, helenalin) [41][35]. Several applications have been described for these compounds, including in the pharmaceutical, fragrance, and food industries [49,50][43][44]. Yeast platforms have been developed for the heterologous production of some of these compounds [51][45].
Diterpenes contain four isoprene units (C20H32) and form linear (phytol), monocyclic (e.g., 9-geranyl-α-terpineol) bicyclic (e.g., sclareol, marrubiin, salvinorin A), tricyclic (e.g., abietic acid, carnosic acid, and tanshinone I), tetracyclic (e.g., gibberellin A1, and steviol) pentacyclic (e.g., wallichanol), or macrocyclic (e.g., casbene, and taxol) structures [41][35]. Polyoxygenated forms with keto and hydroxyl groups (often esterified by small-sized aliphatic or aromatic acids) are commonly found in nature [52][46]. Some diterpenes comprise unique structures, such as ginkgolide B, which is diterpenoid trilactone with six five-membered rings that include a spiro [4,4][4][4]-nonane carbocyclic ring, a tetrahydrofuran ring, and a very specific tert-butyl group at one of the rings, and can be isolated from the tree Ginkgo biloba [53][47]. These compounds exhibit biological activities, such as anti-inflammatory, cardiovascular, antimicrobial, antitumor, antifungal, etc. [54,55][48][49].
Sesterterpenes contain 5 isoprene units with the formula C25H40. They can appear in a wide variety of forms, including linear (e.g., hippolide E), monocyclic (e.g., manoalide), bicyclic (e.g., leucosceptrine), tricyclic (e.g., heliocide H1), tetracyclic (e.g., sesterstatin 7), and macrocyclic (e.g., nitiol, and cybastacines A and B) frameworks. These compounds are synthesized by different organisms, such as marine sponges, plants, fungi, bacteria, or insects. A wide spectrum of biological activities has been found for sesterterpenes, ranging from anti-inflammatory, anticancer, cytotoxic, and antimicrobial, among others [41,56,57][35][50][51].
The carbon skeleton of triterpenes consists of six isoprene units (C30H48). These compounds, which derive from the biosynthesis of squalene, are mainly alcohols, aldehydes, or carboxylic acids, are characterized by the presence of methyl groups. Ursolic acid, betulinic acid, oleanolic acid, pardinols, sitosterol, and campesterol are examples of triterpenoids, which include sterols and phytosterols [41][35]. Oleanolic, ursolic, and betulinic acids are three triterpenic acids with potential effects for the treatment of type 2 diabetes [58][52].
Tetraterpenes comprise eight isoprene units (C40H64) and include the fat-soluble pigments carotenoids (lycopene and β-carotene), and their oxygenated analogues xanthophylls (lutein, zeaxanthin, and astaxanthin). These compounds are mainly present in higher plants and fungi and are characterized by the chromophoric system, a long polyene central chain of conjugated doubled bonds, which provides the characteristic color of these compounds. In fact, carotenoids are responsible for the yellow-orange-red color of plants and animals, the latter incorporating them through diet. Different roles have been assigned to carotenoids, such as antioxidants, as a part of light-harvesting systems, and as photoprotectors of the photosynthetic machinery in photosynthetic organisms. In animals, they constitute a source of vitamin A precursors (retinoids) [59,60,61][53][54][55]. From the industrial point of view, they are used as colorants (several carotenoids are approved and widely used as food colorants [62][56], feed supplements, and nutraceuticals, and they are also interesting for medical, cosmetic, and biotechnological purposes. There is a diversity of natural and synthetic carotenoids, but only a few of them are commercially produced, including carotenes (β-carotene and lycopene) and xanthophylls (astaxanthin, canthaxanthin, lutein, zeaxanthin, and capsanthin). Some biotechnological processes for carotenoids production were established some years ago, but new strains and technologies are being developed nowadays for carotenoids widely in demand [61][55]. Astaxanthin and β-carotene dominate the global market as feed additives and colorants. Two organisms have been developed for the industrial production of β-carotene: the mucor fungus Blakesleea trispora and a halophilic unicellular alga Dunaliella salina, which accumulates high levels of β-carotene as a stress response [63][57]. Several companies and academic laboratories have investigated biological sources of astaxanthin, such as the yeast Xanthophyllomyces dendrorhous (anamorph Phaffia rhodozyma) and the microalga Haematococcus pluvialis, which are able to synthesize high levels of astaxanthin [64][58].
Polyterpenes are polymeric compounds that are formed by more than eight isoprene units and include natural rubbers, which contain isoprene units in the cis-configuration (latex elastomer), and gutta-percha and balata, which contain isoprene units with trans double bonds (inelastic) [41][35].
The classification of terpenes and terpenoids follows the so-called isoprene rule (C5 rule), which is based on the number and organization of carbon atoms in the molecule, thus, grouping these molecules according to the number of isoprene units linked in a head-to-tail manner and constituting hemiterpenes (1 isoprene unit), monoterpenes (2 isoprene units), sesquiterpenes (3 isoprene units), diterpenes (4 isoprene units), etc. (Figure 5). Although the head-to-tail configuration is the most common, non-head-to-tail condensation of isoprene units also exists, such as the head-to-middle fusions occurring in irregular monoterpenoids [36][30]. In addition, isoprene units can conform to chains that can be arranged to form rings.
Hemiterpenes are formed by one isoprene unit. The main hemiterpene is isoprene (methylbuta-1,3-dien, C5H8), a volatile compound commonly found in the atmosphere that results from plant metabolism and is emitted by leaves as a natural byproduct [37,38][31][32]. Isoprene is produced in large amounts by phytoplankton in the oceans, and animals and bacteria can also emit it. Isoprene production in plants appears to be related to the protection of photosynthesis and other processes under stress conditions [39][33]. Plants can synthesize other hemiterpenes, such as several acids (tiglic, angelic, isovaleric, and senecioic) and isoamyl alcohol [40[34][35],41], which are responsible for flavors and aromas.
Monoterpenes comprise two isoprene units (10 carbon atoms; C10H16). These compounds are present in plant oils and are characterized by their strong aroma and odor (used as components of perfumes and cosmetics), and for their biological activity [42,43][36][37]. Monoterpenes can be simple unsaturated hydrocarbons or constitute alcohols, aldehydes, and ketones. They are classified as acyclic or aliphatic (e.g., myrcene, citral, geraniol, lavandulol, and linalool), monocyclic (e.g., α-terpineol, limonene, thymol, menthol, carvone, eucalyptol, and perillaldehyde), and bicyclic, the latter containing a six-member ring and a second ring that can have either three, four, or five members (6 + 3: thujone and Δ3-carene; 6 + 4: α- and β-pinene; 6 + 5: borneol and camphor) [41,44][35][38]. Biosynthesis of α-pinene has been achieved in a genetically engineered Yarrowia lipolytica using low-cost renewable feedstocks [45][39].
A large subtype of monoterpenes is formed by iridoids, which contain a six-membered ring with an oxygen atom fused to a cyclopentane ring (iridane skeleton). They are usually classified as glycosides, since they occur in plants and are often combined with sugars, e.g., aucubin, harpagoside [46][40]. Iridoids are present in medicinal plants and used as sedatives, antipyretics, remedies for wounds, skin disorders, and hypotensives [47][41].
Sesquiterpenes are the most diverse group of terpenoids [48][42]. They consist of three isoprene units (C15H24) and can be naturally found in many latex-producing plants. These compounds are present in linear or acyclic (e.g., farnesol, β-nerolidol), monocyclic (e.g., β-bisabolene, α-zingiberene, α-humulene), bicyclic (e.g., β-santalol, β-caryophyllene, artemisinin), and tricyclic (e.g., khushimol, thujopsene) forms. Sesquiterpenes can also give rise to γ-lactone ring structures (sesquiterpene lactones), thus constituting germacranolides (e.g., costunolide, parthenolide), eudesmanolides (e.g., santonin, alantolactone), and guaianolides (e.g., artabsin, helenalin) [41][35]. Several applications have been described for these compounds, including in the pharmaceutical, fragrance, and food industries [49,50][43][44]. Yeast platforms have been developed for the heterologous production of some of these compounds [51][45].
Diterpenes contain four isoprene units (C20H32) and form linear (phytol), monocyclic (e.g., 9-geranyl-α-terpineol) bicyclic (e.g., sclareol, marrubiin, salvinorin A), tricyclic (e.g., abietic acid, carnosic acid, and tanshinone I), tetracyclic (e.g., gibberellin A1, and steviol) pentacyclic (e.g., wallichanol), or macrocyclic (e.g., casbene, and taxol) structures [41][35]. Polyoxygenated forms with keto and hydroxyl groups (often esterified by small-sized aliphatic or aromatic acids) are commonly found in nature [52][46]. Some diterpenes comprise unique structures, such as ginkgolide B, which is diterpenoid trilactone with six five-membered rings that include a spiro [4,4][4][4]-nonane carbocyclic ring, a tetrahydrofuran ring, and a very specific tert-butyl group at one of the rings, and can be isolated from the tree Ginkgo biloba [53][47]. These compounds exhibit biological activities, such as anti-inflammatory, cardiovascular, antimicrobial, antitumor, antifungal, etc. [54,55][48][49].
Sesterterpenes contain 5 isoprene units with the formula C25H40. They can appear in a wide variety of forms, including linear (e.g., hippolide E), monocyclic (e.g., manoalide), bicyclic (e.g., leucosceptrine), tricyclic (e.g., heliocide H1), tetracyclic (e.g., sesterstatin 7), and macrocyclic (e.g., nitiol, and cybastacines A and B) frameworks. These compounds are synthesized by different organisms, such as marine sponges, plants, fungi, bacteria, or insects. A wide spectrum of biological activities has been found for sesterterpenes, ranging from anti-inflammatory, anticancer, cytotoxic, and antimicrobial, among others [41,56,57][35][50][51].
The carbon skeleton of triterpenes consists of six isoprene units (C30H48). These compounds, which derive from the biosynthesis of squalene, are mainly alcohols, aldehydes, or carboxylic acids, are characterized by the presence of methyl groups. Ursolic acid, betulinic acid, oleanolic acid, pardinols, sitosterol, and campesterol are examples of triterpenoids, which include sterols and phytosterols [41][35]. Oleanolic, ursolic, and betulinic acids are three triterpenic acids with potential effects for the treatment of type 2 diabetes [58][52].
Tetraterpenes comprise eight isoprene units (C40H64) and include the fat-soluble pigments carotenoids (lycopene and β-carotene), and their oxygenated analogues xanthophylls (lutein, zeaxanthin, and astaxanthin). These compounds are mainly present in higher plants and fungi and are characterized by the chromophoric system, a long polyene central chain of conjugated doubled bonds, which provides the characteristic color of these compounds. In fact, carotenoids are responsible for the yellow-orange-red color of plants and animals, the latter incorporating them through diet. Different roles have been assigned to carotenoids, such as antioxidants, as a part of light-harvesting systems, and as photoprotectors of the photosynthetic machinery in photosynthetic organisms. In animals, they constitute a source of vitamin A precursors (retinoids) [59,60,61][53][54][55]. From the industrial point of view, they are used as colorants (several carotenoids are approved and widely used as food colorants [62][56], feed supplements, and nutraceuticals, and they are also interesting for medical, cosmetic, and biotechnological purposes. There is a diversity of natural and synthetic carotenoids, but only a few of them are commercially produced, including carotenes (β-carotene and lycopene) and xanthophylls (astaxanthin, canthaxanthin, lutein, zeaxanthin, and capsanthin). Some biotechnological processes for carotenoids production were established some years ago, but new strains and technologies are being developed nowadays for carotenoids widely in demand [61][55]. Astaxanthin and β-carotene dominate the global market as feed additives and colorants. Two organisms have been developed for the industrial production of β-carotene: the mucor fungus Blakesleea trispora and a halophilic unicellular alga Dunaliella salina, which accumulates high levels of β-carotene as a stress response [63][57]. Several companies and academic laboratories have investigated biological sources of astaxanthin, such as the yeast Xanthophyllomyces dendrorhous (anamorph Phaffia rhodozyma) and the microalga Haematococcus pluvialis, which are able to synthesize high levels of astaxanthin [64][58].
Polyterpenes are polymeric compounds that are formed by more than eight isoprene units and include natural rubbers, which contain isoprene units in the cis-configuration (latex elastomer), and gutta-percha and balata, which contain isoprene units with trans double bonds (inelastic) [41][35].
3. Terpenes and Terpenoids
In the previous section, several types of biomass-derived polymers have been described, important advances have been achieved in the development of biomass-derived plastics, as discussed. However, there is still a long way to go to achieve bio-based plastics that match the properties and uses of petrol-derived polymers. Hence, the search for good bio-based monomer candidates is of paramount importance. Among all the compounds derived from biomass, terpenes have emerged as viable candidates to serve as building blocks for the synthesis of polymers. Terpenes and terpenoids comprise one type of secondary metabolite derived from five-carbon isoprene (isopentane) units (Figure 5), which are assembled in thousands of combinations. Terpenes are merely hydrocarbons, whereas terpenoids (isoprenoids) contain oxidized functional groups. These compounds are ubiquitous in nature since they are naturally produced. Terpenes are produced mainly by plants, where they play a vital role in basic intra- and intercellular processes, such as photosynthetic light reactions, or respiratory chains [29[23][24],30], and they are also components of both primary and secondary metabolism [31][25]. The biosynthesis of terpenoids occurs by means of two independent pathways according to the type of organism, bacteria, archaea, protists, or eukaryotes. The cytosolic mevalonate pathway is functional in archaea, some bacteria, yeast, fungi, and animal cells. On the contrary, the plasticidal 2-C-methyl-D-erythriol 4-phosphate (MEP) pathway is present in most fermentative and aerobic bacteria, photosynthetic bacteria, cyanobacteria, and micro- and macroalgal chloroplasts, as well as all plant chloroplasts [32,33][26][27]. However, many terpenes and terpenoids are produced only in small quantities in their natural sources. Moreover, they are structurally complex, making it difficult to produce them by biochemical synthesis. Therefore, due to the increasing demand of terpenes and terpenoids, much effort is being made to produce these compounds by means of metabolic/genetic engineering technologies and using biorefineries [34][28]. Biotechnological production of terpenoids now offers some major benefits, since microbial production hosts can perform terpenoid biosynthesis from simple carbon sources due to endogenous metabolic pathways, which generates the universal precursors for all terpenoids, namely, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). In addition, current technologies for genome editing allow metabolic engineering of microbial hosts for recombinant terpenoid production [35][29].
Figure 5. Chemical structure of isoprene and classification of terpenes according to the number of isoprene units.
4. Polymerization of Terpenes and Terpenoids
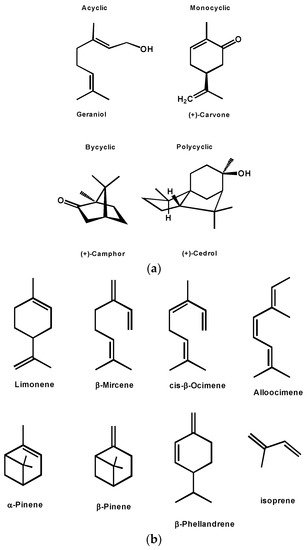
Due to their incredible natural structures, diversity, and different chemo-physical properties, terpenes and terpenoids are molecules of high value. They have a great interest both for traditional and modern applications in the medicine, cosmetics, flavors and fragrance industries, in agriculture, as dietary supplements or food ingredients, also as biomaterials, industrial resins, coatings, and more recently as biofuels [65,66,67][59][60][61]. By no means these multifunctional molecules and are strong candidates to become platform chemicals. However [68[62][63],69], one area that has been less explored is their use as monomers [70][64] to create bio-based polymers and copolymers. This is surprising, as some polyterpenes have been known for a long time such as the case of natural rubber (cis-1,4-polyisoprene) which is a natural terpene-based polymer. The large structural diversity in this family could be the reason for being underexplored as monomers, although, in recent years the number of papers and patents on terpenes and terpenoids polymerization has significantly increased [3,71][3][65]. The polymers obtained from terpenes are predominantly elastomers, while for terpenoids, polymers with a broader range of properties have been described. As well the properties and applications can be significantly enlarged when copolymerized. From a chemical point of view, terpenes are quite close to classical monomers such as ethylene, propylene, and styrene as they also present unsaturated bonds. The ones studied in polymerization can be classified into two main groups: cyclic and acyclic [71,72,73][65][66][67]. Figure 6 shows the most frequently studied within the acyclic group, which are myrcene, ocimene, and alloocimene, while the main cyclic terpenes studied are β-pinene, α-pinene, limonene, and phellandrene. All types of mechanisms have been described for the polymerization of terpenes, as such, these monomers can be polymerized via radical, ionic, coordination-insertion, and coordinative chain-growth mechanisms [74,75,76][68][69][70].
Figure 6. (a) Different classes of terpenes. (b) Common terpenes and terpenoids.
5. Reactive Extrusion to ROP Polymerization
Another key point to progress in the sustainability of the polymers is to develop and improve greener technologies for their production. In this context, reactive extrusion (REX) is becoming an outstanding candidate within green technologies. Moreover, this technology can be applied to different types of polymerizations, which makes it very suitable for application to terpenes and terpenoids where a wide range of mechanisms of polymerization can be applied depending on the chosen precursor.
Reactive extrusion is a very versatile technology that enables not only polymer processing but also polymerizing, grafting, branching, and functionalizing [150][71]. This technique is a low-cost production and processing method for biodegradable plastics.
The advantages of extrusion polymerization are: it is a solvent-free, melt, and continuous process, it is possible to have control over the residence time and distribution, and it can have the integration of other extrusion streams [151][72]. Extruder machines (co-rotating twin-screw extruder) used as a reactor, can be tailored to provide various flow patterns and shear effects, and can thus influence the polymer properties. This is expected to accelerate the kinetics while reducing polydispersity.
However, bulk polymerization has significant kinetic, heat-transfer, and diffusion-related issues that make it, in some cases, difficult to translate the process to reactive extrusion to obtain the high conversion of the monomer and high molecular weight of the polymer [152][73].
From a mechanistic perspective, nearly all kinds of polymerizations have been performed in an extruder. These include radical polymerization, ionic polymerization, metathesis polymerization, and ring-opening polymerization [152][73].
Aliphatic polyesters are usually synthesized by ring-opening polymerization in batch, however, reactive extrusion is a proven and continuous alternative. The synthesis of poly-L-lactide, poly-D,L-lactide, and copolymer Poly-D,L-lactide-co-glycolide by reactive extrusion produces high molecular weights in a controlled way on a time scale of some minutes [148][74]. The catalyst reactive enough to be used to obtain this type of polyesters by reactive extrusion is stannous (II) 2-ethylhexanoate (Sn(Oct)2). This catalyst is typically combined with a protic compound, most frequently a primary alcohol acting as an initiator [153][75]. Triphenylophosphine is also used as a co-catalyst to decrease the side reactions [154][76]. Performing reactive extrusion for these polymers is needed to optimize the transit time within the extruder and control the environment within the extruder regarding the moisture [152][73].
5.1. Reactive Extrusion with Terpenes and Terpenoids
Reactive extrusion with terpenes and terpenoids is very scarce in the literature, in fact, there was an example carried out by Addiego [155][77] where PLA was blended with myrcene and limonene, where these terpenes acted as plasticizers. The main results when comparing conventional and reactive extrusion indicated that myrcene and limonene were efficient plasticizers for PLA, in comparison to neat PLA. Using a free radical initiator during the extrusion of PLA/limonene was beneficial for the mechanical properties, however, it proved detrimental for PLA/myrcene. To our knowledge, REX has not been used to synthesize polyterpenoids, although this synthesis may work in a similar way as the ROP synthesis of PCL and PLA polyesters.
References
- Dupont-Inglis, J.; Borg, A. Destination bioeconomy—The path towards a smarter, more sustainable future. New Biotechnol. 2018, 40, 140–143.
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558.
- Zhu, Y.Q.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362.
- Xu, F. Structure, Ultrastructure, and Chemical Composition. In Cereal Straw as a Resource for Sustainable Biomaterials and Biofuels; Sun, R.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 9–47.
- Duwe, A.; Tippkötter, N.; Ulber, R. Lignocellulose-Biorefinery: Ethanol-Focused. In Biorefineries; Advances in Biochemical Engineering/Biotechnology; Springer: Cham, Switzerland, 2017; pp. 177–216.
- Tajmirriahi, M.; Momayez, F.; Karimi, K. The critical impact of rice straw extractives on biogas and bioethanol production. Bioresour. Technol. 2021, 319, 124167.
- de Paula, R.G.; Antoniêto, A.C.; Ribeiro, L.F.; Srivastava, N.; O’Donovan, A.; Mishra, P.K.; Gupta, V.K.; Silva, R.N. Engineered microbial host selection for value-added bioproducts from lignocellulose. Biotechnol. Adv. 2019, 37, 107347.
- Machado, G.; Santos, F.; Lourega, R.; Mattia, J.; Faria, D.; Eichler, P.; Auler, A. Biopolymers from Lignocellulosic Biomass (Feedstocks, Production Processes, and Applications); Lignocellulosic Biorefining Technologies; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 125–158.
- Bela Torok, T.D. Green Chemistry, An Inclusive Approach; Elsevier: Cambridge, MA, USA, 2018.
- Shen, L.; Worrell, E.; Patel, M. Present and future development in plastics from biomass. Biofuels Bioprod. Biorefin. Innov. Sustain. Econ. 2010, 4, 25–40.
- Narancic, T.; Cerrone, F.; Beagan, N.; O’Connor, K.E. Recent Advances in Bioplastics: Application and Biodegradation. Polymers 2020, 12, 920.
- UNE-CEN/TR 15932:2010 IN: Recommendation for Terminology and Characterisation of Biopolymers and Bioplastics. Available online: https://infostore.saiglobal.com/en-gb/Standards/CEN-TR-15932-2010-335831_SAIG_CEN_CEN_770974/ (accessed on 5 March 2021).
- Fact Sheet, European Bioplastics: What are the Bioplastics? Available online: https://docs.european-bioplastics.org/publications/fs/EuBP_FS_What_are_bioplastics.pdf (accessed on 3 February 2021).
- Michael Thielen, B.M. Fachagentur Nachwachsende Rohstoffe e.V. (FNR); Agency for Renewable Resources: Gülzow, Germany, 2014.
- Benavides, P.T.; Lee, U.; Zare-Mehrjerdi, O. Life cycle greenhouse gas emissions and energy use of polylactic acid, bio-derived polyethylene, and fossil-derived polyethylene. J. Clean. Prod. 2020, 277, 124010.
- Batori, V.; Akesson, D.; Zamani, A.; Taherzadeh, M.J.; Horvath, I.S. Anaerobic degradation of bioplastics: A review. Waste Manag. 2018, 80, 406–413.
- Arikan, E.B.; Ozsoy, H.D. A Review: Investigation of Bioplastics. J. Civ. Eng. Arch. 2015, 9, 188–192.
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.-B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martínez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-Based Packaging: Materials, Modifications, Industrial Applications and Sustainability. Polymers 2020, 12, 1558.
- Bioplastics Market Data. Available online: https://www.european-bioplastics.org (accessed on 5 March 2021).
- Nakajima, H.; Dijkstra, P.; Loos, K. The Recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers that Are Upgraded from Biodegradable Polymers, Analogous to Petroleum-Derived Polymers, and Newly Developed. Polymers 2017, 9, 523.
- Biopolymers. Available online: https://www.eubia.org/cms/wiki-biomass/bio-based-products/biopolymers/ (accessed on 5 March 2021).
- Imre, B.; Pukanszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233.
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414.
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. In Biotechnology of Isoprenoids; Schrader, J., Bohlmann, J., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin, Germany, 2015; Volume 148, pp. 63–106.
- Kännaste, A.; Copolovici, L.; Niinemets, Ü. Plant Isoprenoids: Methods and Protocols, Methods in Molecular Biology; Springer: New York, NY, USA, 2014; pp. 1–5.
- Lombard, J.; Moreira, D. Origins and Early Evolution of the Mevalonate Pathway of Isoprenoid Biosynthesis in the Three Domains of Life. Mol. Biol. Evol. 2011, 28, 87–99.
- Lohr, M.; Schwender, J.; Polle, J.E. Isoprenoid biosynthesis in eukaryotic phototrophs: A spotlight on algae. Plant Sci. 2012, 185, 9–22.
- Schrader, J.; Bohlmann, J. (Eds.) Biotechnology of Isoprenoids; Springer: Berlin, Germany, 2015.
- Moser, S.; Pichler, H. Identifying and engineering the ideal microbial terpenoid production host. Appl. Microbiol. Biotechnol. 2019, 103, 5501–5516.
- Connolly, J.D.; Hill, R.A. Dictionary of Terpenoids; Chapman & Hall: London, UK, 1991.
- Croteau, R.O.; Johnson, M.A. Biosynthesis and Biodegradation of Wood Components; Academic Press: Cambridge, MA, USA, 1985; pp. 379–439.
- Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, C.; Palmer, P.I.; Geron, C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos. Chem. Phys. 2006, 6, 3181–3210.
- Ryan, A.C.; Hewitt, C.N.; Possell, M.; Vickers, C.E.; Purnell, A.; Mullineaux, P.M.; Davies, W.J.; Dodd, I.C. Isoprene emission protects photosynthesis but reduces plant productivity during drought in transgenic tobacco (Nicotiana tabacum) plants. New Phytol. 2014, 201, 205–216.
- Cseke, L.J.; Kirakosyan, A.; Kaufman, P.B.; Warber, S.; Duke, J.A.; Brielmann, H.L. Natural Products from Plants; Taylor and Francis: London, UK, 2006.
- Ludwiczuk, A.S.; Georgiev, M.I. Chapter 11: Terpenoids. In Pharmacognosy; Boston Academic: Boston, MA, USA, 2017; pp. 233–266.
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478.
- Sobral, M.V.; Xavier, A.L.; Lima, T.C.; de Sousa, D.P. Antitumor activity of monoterpenes found in essential oils. Sci. World J. 2014, 2014, 953451.
- Singh, G. Chemistry of Terpenoids and Carotenoids; Discovery: New Delhi, India, 2007.
- Wei, L.J.; Zhong, Y.T.; Nie, M.Y.; Liu, S.C.; Hua, Q. Biosynthesis of alpha-Pinene by Genetically Engineered Yarrowia lipolytica from Low-Cost Renewable Feedstocks. J. Agric. Food. Chem. 2021, 69, 275–285.
- Villasenor, I.M. Bioactivities of iridoids. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2007, 6, 307–314.
- Dinda, B.; Debnath, S.; Harigaya, Y. Naturally occurring iridoids. A review, part 1. Chem. Pharm. Bull. 2007, 55, 159–222.
- Misawa, N. Pathway engineering for functional isoprenoids. Curr. Opin. Biotechnol. 2011, 22, 627–633.
- Chan, W.K.; Tan, L.T.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529.
- de Veras, B.O.; de Oliveira, M.B.; da Silva Oliveira, F.G.; Dos Santos, Y.Q.; de Oliveira, J.R.; de Menezes Lima, V.L.; da Silva Almeida, J.R.; Navarro, D.M.; de Oliveira Farias, J.C.; dos Santos Aguiar, J.; et al. Chemical composition and evaluation of the antinociceptive, antioxidant and antimicrobial effects of essential oil from Hymenaea cangaceira (Pinto, Mansano & Azevedo) native to Brazil: A natural medicine. J. Ethnopharmacol. 2020, 247, 112265.
- Kutyna, D.R.; Borneman, A.R. Heterologous Production of Flavour and Aroma Compounds in Saccharomyces cerevisiae. Genes 2018, 9, 326.
- Ramawat, K.G.; Mérillon, J.M. Diterpenes for Therapeutic Use. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3171–3191.
- Stromgaard, K.; Nakanishi, K. Chemistry and biology of terpene trilactones from Ginkgo biloba. Angew. Chem. Int. Ed. 2004, 43, 1640–1658.
- Topcu, G.; Gören, A.C. Biological Activity of Diterpenoids Isolated from Anatolian Lamiaceae Plants. Rec. Nat. Prod. 2007, 1, 1–16.
- Guerra-Bubb, J.; Croteau, R.; Williams, R.M. The early stages of taxol biosynthesis: An interim report on the synthesis and identification of early pathway metabolites. Nat. Prod. Rep. 2012, 29, 683–696.
- Liu, Y.; Wang, L.; Jung, J.H.; Zhanga, S. Sesterterpenoids. Nat. Prod. Rep. 2007, 24, 1401–1429.
- Ebada, S.S.; Lin, W.; Proksch, P. Bioactive Sesterterpenes and Triterpenes from Marine Sponges: Occurrence and Pharmacological Significance. Mar. Drugs 2010, 8, 313–346.
- Silva, F.S.; Oliveira, P.J.; Duarte, M.F. Oleanolic, Ursolic, and Betulinic Acids as Food Supplements or Pharmaceutical Agents for Type 2 Diabetes: Promise or Illusion? J. Agric. Food. Chem. 2016, 64, 2991–3008.
- Blomhoff, R.; Blomhoff, H.K. Overview of retinoid metabolism and function. J. Neurobiol. 2006, 66, 606–630.
- Domonkos, I.; Kis, M.; Gombos, Z.; Ughy, B. Carotenoids, versatile components of oxygenic photosynthesis. Prog. Lipid Res. 2013, 52, 539–561.
- Barredo, J.L.; Garcia-Estrada, C.; Kosalkova, K.; Barreiro, C. Biosynthesis of Astaxanthin as a Main Carotenoid in the Heterobasidiomycetous Yeast Xanthophyllomyces dendrorhous. J. Fungi 2017, 3, 44.
- Mortensen, A. Carotenoids and other pigments as natural colorants. Pure Appl. Chem. 2006, 78, 1477–1491.
- Nonomura, A.M. Industrial biosynthesis of carotenoids. In Carotenoids: Chemistry and Biology; Plenum Press: New York, NY, USA, 1989; pp. 365–375.
- Rodriguez-Saiz, M.; Luis de la Fuente, J.; Luis Barredo, J. Xanthophyllomyces dendrorhous for the industrial production of astaxanthin. Appl. Microbiol. Biotechnol. 2010, 88, 645–658.
- Bohlmann, J.; Keeling, C.I. Terpenoid biomaterials. Plant J. 2008, 54, 656–669.
- Zerbe, P.; Bohlmann, J. Recent Advances in Phytochemistry. In Phytochemicals—Biosynthesis, Function and Application; Springer: Berlin, Germany, 2014; Volume 44, pp. 85–108.
- Tetali, S.D. Terpenes and isoprenoids: A wealth of compounds for global use. Planta 2019, 249, 1–8.
- Kuehlborn, J.; Gross, J.; Opatz, T. Making natural products from renewable feedstocks: Back to the roots? Nat. Prod. Rep. 2020, 37, 380–424.
- Behr, A.; Johnen, L. Myrcene as a Natural Base Chemical in Sustainable Chemistry: A Critical Review. Chemsuschem 2009, 2, 1072–1095.
- Palenzuela, M.; Sánchez-Roa, D.; Damián, J.; Sessini, V.; Mosquera, M.E. Polymerization of terpenes and terpenoids using metal catalysts. Adv. Organomet. Chem. 2021, 75, 55–93.
- Winnacker, M.; Rieger, B. Recent Progress in Sustainable Polymers Obtained from Cyclic Terpenes: Synthesis, Properties, and Application Potential. Chemsuschem 2015, 8, 2455–2471.
- Swift, K.A. Catalytic transformations of the major terpene feedstocks. Top. Catal. 2004, 27, 143–155.
- Sarkar, P.; Bhowmick, A.K. Synthesis, characterization and properties of a bio-based elastomer: Polymyrcene. RSC Adv. 2014, 4, 61343–61354.
- Aoshima, S.; Kanaoka, S. A Renaissance in Living Cationic Polymerization. Chem. Rev. 2009, 109, 5245–5287.
- Georges, S.; Toure, A.O.; Visseaux, M.; Zinck, P. Coordinative Chain Transfer Copolymerization and Terpolymerization of Conjugated Dienes. Macromolecules 2014, 47, 4538–4547.
- Avila-Ortega, A.; Aguilar-Vega, M.; Bastarrachea, M.I.; Carrera-Figueiras, C.; Campos-Covarrubias, M. Anionic synthesis of amine omega-terminated beta-myrcene polymers. J. Polym. Res. 2015, 22, 226.
- Mani, R.; Bhattacharya, M.; Tang, J. Functionalization of polyesters with maleic anhydride by reactive extrusion. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 1693–1702.
- Raquez, J.M.; Degee, P.; Nabar, Y.; Narayan, R.; Dubois, P. Biodegradable materials by reactive extrusion: From catalyzed polymerization to functionalization and blend compatibilization. C. D. Chim. 2006, 9, 1370–1379.
- Fink, J.K. Reactive Polymers: Fundamentals and Applications. A Concise Guide to Industrial Polymers; Plastics Design Library; Elsevier: Amsterdam, The Netherlands, 2018; pp. 449–496.
- Anderson, T.S.; Kozak, C.M. Ring-opening polymerization of epoxides and ring-opening copolymerization of CO2 with epoxides by a zinc amino-bis(phenolate) catalyst. Eur. Polym. J. 2019, 120, 109237.
- Raquez, J.M.; Ramy-Ratiarison, R.; Murariu, M.; Dubois, P. Reactive Extrusion of PLA-based Materials: From Synthesis to Reactive melt-blending. Technol. Process. Prop. Addit. Appl. R. Soc. Chem. Polym. Chem. Ser. 2014, 101–122.
- Jacobsen, S.; Fritz, H.G.; Degee, P.; Dubois, P.; Jerome, R. Single-step reactive extrusion of PLLA in a corotating twin-screw extruder promoted by 2-ethylhexanoic acid tin(II) salt and triphenylphosphine. Polymer 2000, 41, 3395–3403.
- Bruester, B.; Adjoua, Y.O.; Dieden, R.; Grysan, P.; Federico, C.E.; Berthe, V.; Addiego, F. Plasticization of Polylactide with Myrcene and Limonene as Bio-Based Plasticizers: Conventional vs. Reactive Extrusion. Polymers 2019, 11, 1363.
More
