Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Zhongling Shang and Version 2 by Camila Xu.
As an apoplastic signal, extracellular ATP (eATP) promotes pollen germination (PG) and pollen tube growth (PTG) of Arabidopsis thaliana by stimulating Ca2+ or K+ absorption. P2K1 receptor, heterotrimeric G alpha protein and CNGC2/CNGC4 are involved in eATP stimulated signaling in Arabidopsis pollens.
- extracellular ATP (eATP)
- Arabidopsis thaliana
- pollen grain
- ion influx
- signaling
1. Introduction
The apoplast, including the cell wall and intercellular space, plays essential roles in modulating plant cell growth and development due to the existence of numerous signaling molecules within the apoplastic matrix. In recent years, growing evidence has shown that adenosine triphosphate (ATP) is present in the apoplast and participates in physiological processes such as vegetative growth, development, and stress response [1][2][3][4][1,2,3,4]. eATP is involved in maintaining cell viability [5] and the growth rate of cultured cells [6], regulating the rate or direction of the growth of roots [7], hypocotyls [8][9][8,9], and cotton fibers [10]. It is also involved in modulating stomatal movement [11][12][13][11,12,13]. Some stresses induce eATP release as a “danger signal”, which in turn induces resistance responses to disease, hypotonic conditions, high salinity, and cold stress [14][15][16][17][18][14,15,16,17,18].
The signal transduction of eATP has been intensively investigated over the past two decades. Two lectin receptor kinases, P2K1 and P2K2, were identified to be eATP receptors in Arabidopsis thaliana [19][20][19,20] involved in eATP-induced immune responses [13][14][21][13,14,21]. Signal transducers in the plasma membrane (PM), including heterotrimeric G proteins [12][22][12,22], NADPH oxidase [23][24][23,24], and Ca2+ channels [6][23][25][26][6,23,25,26], were reported to be involved in eATP signal transduction. Several secondary messengers, e.g., ROS, H2O2, NO, and Ca2+ may be responsible for the eATP-induced intracellular responses that eventually alter plant growth and development [6][17][26][27][28][29][6,17,26,27,28,29].
PG and PTG are fundamental processes in plant sexual reproduction. Ion intake is the main factor affecting the two processes [30][31][32][30,31,32]. Through the regulation of turgor pressure, K+ ions play essential roles in PG and PTG. Proper K+ channel expression is essential for Arabidopsis pollen hydration on stigma [33]. K+ influx causes pollen tube apex bursting and the release of sperm cells to complete double fertilization [34] and endows pollen with competitive ability [35]. Excessively low or high K+ or Ca2+ concentration inhibited PG and PTG in vitro in Arabidopsis [36][37][36,37]. Inward K+ channels that drive K+ absorption by pollen cells are involved in PG and PTG [33][38][39][40][33,38,39,40]. Ca2+ is involved in modulating the activity of some inward K+ channels in pollen protoplasts [39][41][39,41].
As a component of the cell wall and a cytoplasmic messenger, Ca2+ ions play essential roles in PG and PTG [31][32][42][43][44][45][31,32,42,43,44,45]. Sufficient Ca2+ is necessary for PG and PTG. Pollen tubes emerge from the aperture where high cytosolic Ca2+ concentration ([Ca2+]cyt) is localized [42][46][47][42,46,47]. [Ca2+]cyt initiates pollen germination [46] and regulates the rate and direction of growth of pollen tubes [44][45][48][49][50][51][44,45,48,49,50,51]. Exocytosis occurs continuously during PG and PTG. Ca2+-regulated vesicle trafficking and fusion with the PM therefore play key roles in PG and PTG [51][52][53][54][55][56][57][51,52,53,54,55,56,57]. Ca2+ influx and signaling are responsible for stimuli-triggered or -regulated PG and PTG [58][59][60][61][62][63][58,59,60,61,62,63].
2. ATP Addition Impacts PG and PTG in Pollens from 34 Species
To verify the universality of eATP-regulated PG and PTG, we examined the effects of 0.1 mM ATP on PG and PTG of pollen grains from 34 species. Pollen germination rates increased significantly in 28 species (p < 0.05) and decreased significantly in 6 species (p < 0.05). Pollen tube length measurement results showed that tube growth was significantly promoted in 19 species (p < 0.05) and significantly inhibited in 5 species, while no effect was observed in 10 species (Figure 1).
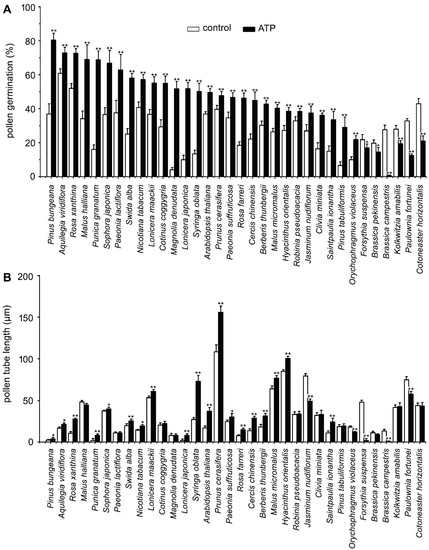

Figure 1. ATP-regulated PG and PTG in various plant species. Pollen grains of 34 plant species were germinated in vitro in the medium containing 18% sucrose, 0.1 mM KCl, and 0.5% agarose. Germination duration of each species is listed in Table 1. PG (A) and PTG (B) were noted. In each experiment, at least 300 pollen grains or 150 pollen tubes were counted or measured. Data from 3 replicates were calculated to obtain mean ± SD. Student’s t-test p values: * p < 0.05, ** p < 0.01.
Table 1.
Plant materials for in vitro pollen germination (sorted by the initials of the Latin names of plant species).
| Latin Name | Family | Class | Phylum | Germination Duration (min) | |
|---|---|---|---|---|---|
| 1 | Aquilegia viridiflora | Ranunculaceae | Dicotyledoneae | Angiospermae | 30 |
| 2 | Arabidopsis thaliana | Brassicaceae | Dicotyledoneae | Angiospermae | 300 |
| 3 | Berberis thunbergii | Berberidaceae | Dicotyledoneae | Angiospermae | 40 |
| 4 | Brassica campestris | Brassicaceae | Dicotyledoneae | Angiospermae | 240 |
| 5 | Brassica pekinensis | Brassicaceae | Dicotyledoneae | Angiospermae | 150 |
| 6 | Cercis chinensis | Leguminosae | Dicotyledoneae | Angiospermae | 90 |
| 7 | Clivia miniata | Amaryllidaceae | Monocotyledoneae | Angiospermae | 420 |
| 8 | Cotinus coggygria | Anacardiaceae | Dicotyledoneae | Angiospermae | 40 |
| 9 | Cotoneaster horizontalis | Rosaceae | Dicotyledoneae | Angiospermae | 60 |
| 10 | Forsythia suspensa | Oleaceae | Dicotyledoneae | Angiospermae | 120 |
| 11 | Hyacinthus orientalis | Hyacinthaceae | Monocotyledoneae | Angiospermae | 80 |
| 12 | Jasminum nudiflorum | Oleaceae | Dicotyledoneae | Angiospermae | 90 |
| 13 | Kolkwitzia amabilis | Caprifoliaceae | Dicotyledoneae | Angiospermae | 60 |
| 14 | Lonicera maackii | Caprifoliaceae | Dicotyledoneae | Angiospermae | 60 |
| 15 | Lonicera japonica | Caprifoliaceae | Dicotyledoneae | Angiospermae | 120 |
| 16 | Magnolia denudata | Magnoliaceae | Dicotyledoneae | Angiospermae | 2880 |
| 17 | Malus halliana | Rosaceae | Dicotyledoneae | Angiospermae | 30 |
| 18 | Malus micromalus | Rosaceae | Dicotyledoneae | Angiospermae | 60 |
| 19 | Nicotiana tabacum | Solanaceae | Dicotyledoneae | Angiospermae | 30 |
| 20 | Orychophragmus violaceus | Brassicaceae | Dicotyledoneae | Angiospermae | 180 |
| 21 | Paeonia suffruticosa | Paeoniaceae | Dicotyledoneae | Angiospermae | 60 |
| 22 | Paeonia lactiflora | Paeoniaceae | Dicotyledoneae | Angiospermae | 60 |
| 23 | Paulownia fortunei | Scrophulariaceae | Dicotyledoneae | Angiospermae | 60 |
| 24 | Pinus bungeana | Pinaceae | Coniferopsida | Gymnosperm | 4320 |
| 25 | Pinus tabulaeformis | Pinaceae | Coniferopsida | Gymnosperm | 4320 |
| 26 | Punica granatum | Punicaceae | Dicotyledoneae | Angiospermae | 120 |
| 27 | Prunus cerasifera | Rosaceae | Dicotyledoneae | Angiospermae | 60 |
| 28 | Rosa xanthina | Rosaceae | Dicotyledoneae | Angiospermae | 20 |
| 29 | Robinia pseudoacacia | Leguminosae | Dicotyledoneae | Angiospermae | 30 |
| 30 | Rosa farreri | Rosaceae | Dicotyledoneae | Angiospermae | 30 |
| 31 | Saintpaulia ionantha | Gesneriaceae | Dicotyledoneae | Angiospermae | 180 |
| 32 | Sophora japonica | Leguminosae | Dicotyledoneae | Angiospermae | 30 |
| 33 | Swida alba | Corneceae | Dicotyledoneae | Angiospermae | 60 |
| 34 | Syringa oblata | Oleaceae | Dicotyledoneae | Angiospermae | 90 |
3. eATP Regulates PG and PTG of Arabidopsis thaliana via K+ and Ca2+ Intake
To verify the role of ion uptake in eATP-promoted PG and PTG, Arabidopsis thaliana pollen was germinated in vitro, and the effects of apyrase or ATP addition on PG and PTG were investigated. In the basic medium, which contained 0.1 mM KCl, apyrase inhibited PG and PTG significantly. After 100 units/mL apyrase treatment, the pollen germination rate decreased from 39.2 ± 2.5% to 26.7 ± 3.4%, and the pollen tube length decreased from 16.9 ± 2.4 μm to 10.3 ± 1.8 μm (p < 0.05). Control treatment with heat-denatured apyrase or bovine serum albumin (BSA) did not affect PG or PTG (p > 0.05) (Figure 2A,D). At low K+ concentrations (0.02–0.1 mM), ATP supplementation (0.1 mM) promoted PG and PTG significantly, while at high K+ concentration (1.0 mM), ATP supplementation slightly inhibited PG but did not affect PTG (Figure 2B,E). To confirm that eATP supplementation promotes PG and PTG by increasing K+ uptake, 1 mM of the K+ channel blockers Ba2+, Cs+, or TEA (tetraethylammonium) was added into the basic medium containing 0.1 mM KCl. In these conditions, the effects of eATP on PG or PTG were significantly suppressed (p < 0.01) (Figure 2C,F).
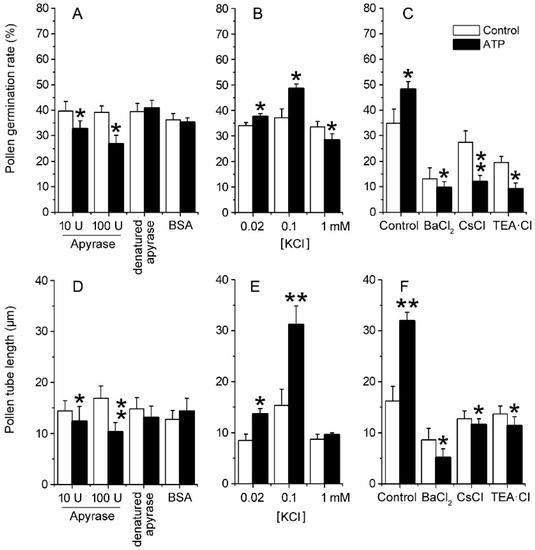

Figure 2. eATP regulates the PG and PTG of Arabidopsis thaliana by facilitating K+ uptake. Apyrase-inhibited PG (A) and PTG (D), ATP-regulated PG (B), and PTG (E) in medium containing serial concentrations of KCl. K+ channel blockers (1 mM) inhibited ATP-promoted PG (C) and PTG (F). In (A–F), the K+ concentration in the medium is 0.1 mM. In each experiment, at least 300 pollen grains were counted to obtain PG, and at least 150 pollen tubes were measured to obtain the pollen tube length. Data from 3 replicates were combined to obtain the mean ± SD. Student’s t test p values: * p < 0.05, ** p < 0.01.
To verify the role of Ca2+ in eATP-promoted PG and PTG, 0.1 mM ATP was added to medium containing EGTA, series of concentrations of Ca2+, or Ca2+ channel blockers. In 0.5 mM EGTA-containing medium, PG and PTG were significantly suppressed, and ATP supplementation did not have any effect. In medium containing 0.1 mM CaCl2, PG and PTG were both stimulated by 0.1 mM ATP. In medium containing 1 mM CaCl2, pollen tube growth was markedly stimulated, while pollen germination was suppressed by 0.1 mM ATP. In medium containing 0.1 mM Ca2+-permeable channel blockers (Gd3+ or La3+), resting PG and PTG were both suppressed, and added ATP did not have any effect on PG and PTG (Figure 3). These data indicate that eATP may promote PG and PTG by facilitating Ca2+ intake.
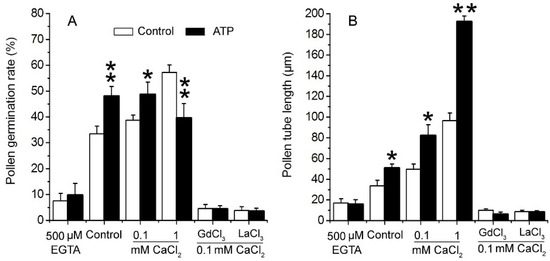

Figure 3. eATP regulates PG and PTG of Arabidopsis thaliana by facilitating Ca2+ uptake. ATP (0.1 mM) regulates PG (A) and PTG (B) in medium containing a Ca2+ chelator, serial concentrations of Ca2+, and channel blockers (0.1 mM). In each experiment, at least 300 pollen grains were counted to obtain PG, and at least 150 pollen tubes were measured to obtain the pollen tube length. Data from 3 replicates were combined to obtain the mean ± SD. Student’s t test p values: * p < 0.05, ** p < 0.01.
To verify the role of ATP hydrolytes in PG and PTG, 0.1 mM ADP, AMP, adenosine, adenine, or phosphate (Pi) was added to the medium containing 0.1 mM KCl or CaCl2. PG and PTG were not affected by these reagents in either KCl or CaCl2 medium (Figure S1A,C). To verify the effect of various ATP salts on PG and PTG, 0.1 mM ATP sodium, Tris, or magnesium salt was added to the medium containing 0.1 mM KCl or CaCl2. PG and PTG were significantly promoted by the three ATP salts (p < 0.05) in both the KCl and CaCl2 media (Figure S1B–D). To ensure that ATP acts as a signal rather than an energy carrier, 0.1 mM ATPγS, a weakly-hydrolyzable ATP analog, was added to the medium containing either 0.1 mM KCl or CaCl2. PG and PTG were significantly promoted by ATPγS (p < 0.05) in the KCl and CaCl2 media (Figure S1B–D).
4. ATP Stimulates K+ and Ca2+ Influx in Arabidopsis thaliana Pollen Protoplast
To confirm that eATP stimulates K+ uptake in pollen grains, whole-cell patch clamping was performed to detect the effect of eATP on K+ conductance in the PM. In pollen protoplasts, a time-dependent inward-rectifying K+ current was recorded at a negative voltage of more than −140 mV. The addition of 1 mM CsCl significantly suppressed the current intensity, confirming that the inward currents may be carried by K+ influx (Figure 4A). The addition of 0.1 mM ATP stimulated the inward K+ current: the maximum current intensity increased from −129 ± 32 pA to −254 ± 34 pA at −200 mV (n = 7, p < 0.05), and the open potential shifted to more positive (Figure 4B). In CsCl-pretreated protoplasts, the effect of ATP on the inward K+ currents was markedly suppressed. The maximum current intensity decreased from −270 ± 35 pA to −83 ± 18 pA at −200 mV (n = 7, p < 0.05) (Figure 4C).
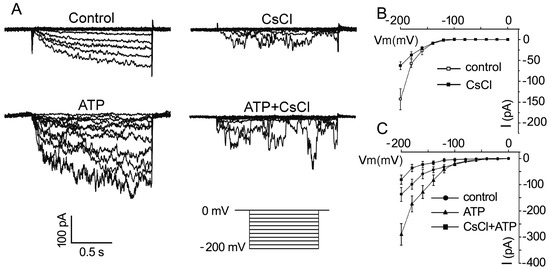

Figure 4. CsCl inhibits eATP-stimulated K+ influx in the protoplasts of Arabidopsis pollen grains. (A) CsCl (1 mM) inhibited resting and 0.1 mM ATP-stimulated inward K+ currents. Representative current traces from step-voltage clamping are shown. (B,C) I-V relationship curves of inward K+ currents (n = 7). In each experiment, data from 7 protoplasts were recorded and combined to obtain the mean ± SD.
To further confirm the promotion of eATP on the Ca2+ uptake of pollen grains, the effect of 0.1 mM ATP on inward Ca2+ conductance was investigated. The addition of 0.1 mM ATP strongly promoted Ca2+ influx in pollen protoplasts: the maximum inward current intensity at −200 mV increased from −147.6 ± 21.9 pA to −243.1 ± 22.8 pA (n = 7, p < 0.05) (Figure 5A,B). GdCl3 (1 mM) significantly suppressed Ca2+ conductance (Figure 5A,C, n = 7, p < 0.05). In Gd3+-pretreated pollen protoplasts, ATP did not stimulate Ca2+ inward conductance (Figure 5A,C, n = 7, p > 0.05).
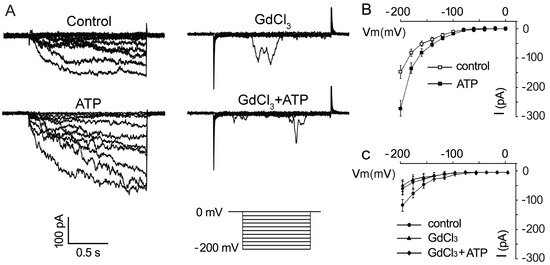

Figure 5. GdCl3 inhibits eATP-stimulated Ca2+ influx in the protoplasts of Arabidopsis pollen grain. (A) GdCl3 (0.1 mM) inhibited resting and 0.1 mM ATP-stimulated inward Ca2+ currents. Representative current traces from step-voltage clamping are shown. (B,C) I-V relationship curves of the inward Ca2+ currents (n = 7). In each experiment, data from 7 protoplasts were recorded and combined to obtain the mean ± SD.
