Epithelial ovarian cancer (EOC) is one of the most common causes of cancer-related deaths among women and is associated with age and age-related diseases. With increasing evidence of risks associated with metabolic inflammatory conditions, such as obesity and type 2 diabetes mellitus (T2DM), it is important to understand the complex pathophysiological mechanisms underlying cancer progression and metastasis. Age-related conditions can lead to both genotypic and phenotypic immune function alterations, such as induction of senescence, which can contribute to disease progression.
- epithelial ovarian cancer
- milky spots
- senescence
- T cells
- diabetes mellitus
1. Introduction
Epithelial ovarian cancer (EOC) is the eighth most common cause of cancer-related deaths among women globally, with a survival rate of less than 50% and an average age at diagnosis of 63 years [1,2][1][2]. The poor prognosis of ovarian cancers, which lack symptoms at an early stage, means that 80% of all cases are diagnosed at a later, more advanced stage [1,3][1][3]. With age and age-related disorders, such as diabetes, acting as significant risk factors for disease progression in EOC, it raises the question of which element of the ageing process is leading to such a change in prognosis [2]. With many of the components of the omentum changing with age, including a loss of the basement membrane, which aids tumour invasion, and distinct changes to mesothelial cells, the peritoneal extracellular matrix, immune cells, and adipocytes [2], a concept that seems to be key to increased inflammation associated with ageing is termed cellular senescence. Accumulation of senescent cells in age-related disease, such as diabetes, is known to create a proinflammatory environment for disease progression [2,4][2][4]. Nevertheless, the recent discovery of increased numbers of senescent T cells in type 2 diabetic patients may indicate a role of senescent immune cells as potential contributors to the progression and advancements in EOC metastasis [5].
A number of risk factors and disorders, such as ageing, smoking, late pregnancy, diabetes, and obesity, have been reported to be associated with poor survival of ovarian cancer patients. For instance, in recent years, type 2 diabetes mellitus (T2DM) has been identified as an independent risk factor for mortality in patients with EOC. Bakhru et al. (2011), in their observational study of 642 patients, revealed a decrease in overall survival (OS) from 6 years for nondiabetic patients to 4 years for diabetic patients with EOC [6]. A retrospective cohort study (n = 367) confirmed these findings, with diabetic patients exhibiting both poorer progression-free survival (PFS) (10.3 vs. 16.3 months) and OS (26.1 vs. 42.2 months) [7]. The association between T2DM and EOC is complex as there are multifactorial aspects, but also the molecular basis of this remains unclear. Due to these pathophysiological features, diabetes is associated with a chronic, low-grade systemic inflammation, which is believed to be linked with cellular senescence, particularly in the lymphocytes and macrophages [8,9,10][8][9][10]. According to early studies, this persistent low-grade inflammation in T2DM was believed to be caused by macrophages, which led many groups to concentrate on the role of innate immunity in disease development. However, now it has been discovered that the number of senescent T cells (both CD4 + and CD8 + ) increases in patients with T2DM and is believed to be a contributory factor to disease progression [11].
Another condition that contributes to EOC progression is obesity. One of the biggest studies, “The Million Women Study” based in the United Kingdom, followed 1.2 million women for an average of 5.4 years for cancer incidence and 7.0 years for cancer mortality. This study found that women with a BMI ≥ 25 have a higher incidence of EOC compared with their normal weight counterparts/women with a BMI of 18.5–24.9 [12]. Nagle et al. (2015) studied the associations between histopathological types of EOC and disease outcomes in patients with obesity, showing increased mortality in cases of high- and low-grade serous and endometrioid cancers [13]. In ovarian cancer patients, obesity induces chronic inflammation that can modify the tumour microenvironment and induce T cell senescence [14[14][15][16],15,16], which could potentially contribute to EOC progression. Chronic inflammation has been shown to involve a chemokine network that influences the migration and invasion of cancer cells, which support the tumour microenvironment for cancer progression by increasing the inflammatory burden [17,18][17][18]. This chronic inflammation has been strongly associated with inducing senescence, particularly immune senescence in age-related metabolic disorders, such as T2DM, and in ovarian cancer [11,19][11][19]. Given that chronic-inflammation-induced senescence is a detrimental factor associated with poor prognosis in T2DM and obesity comorbidities, there is a possibility that these immune cells in the ageing peritoneum of T2DM patients contribute to creating a more proinflammatory, tumour-friendly premetastatic niche for migrating EOC cells to form secondary foci. Subsequently, this cascade could lead to metastasis. In this review, we will explore this hypothesis in the light of immune senescence in “milky spots” and secondary ovarian tumour growth and metastasis in the omentum.
2. Omental Milky Spots as the Preferential Site for Colonisation by Epithelial Cancer Cells
The omentum is the preferred site for peritoneal cancer cells to colonise and proliferate [3] and is the most common site of EOC metastasis [1] ( Figure 1 ). The omentum is highly vascularised and formed as the parietal peritoneum folds, lying between the parietal peritoneum and the anterior surface of the abdominal organs. Structurally, the omentum has a matrix that is rich in collagen with a mesothelial cell layer and a thin basement membrane [2]. It shows two contrasting structural regions—one with a collagenous membranelike organisation and the other rich in adipose tissue—accommodating blood vessels, lymph nodes, and clusters of immune cells [20]. The healthy omentum plays a key role in repairing injury, fighting infections, and releasing factors to aid local homeostasis [3]. It is also known to have a significant impact on peritoneal immunity due to the high number of lymphoid clusters or “milky spots” present [2].
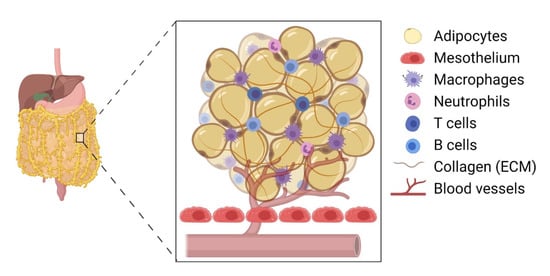
Clark et al. (2013) provided compelling evidence that EOC cells preferentially colonise the milky spots in the omental tissue compared with other peritoneal tissues [35][21]. In their experiment, both C57BL/6 and nude mice were injected (intraperitoneally) with EOC cell lines, such as ID8, SKOV3, CaOV3, and HeyA8 cells. Interestingly, their histological analysis revealed 48 foci of cancer cells within the omentum and only 5 foci in the splenoportal fat [35][21]. There were no ovarian cancer cells detected in the uterine or gonadal fat, indicating a milky-spot-specific directed migration of EOC cells within the peritoneum. For example, cancer cell migration increased by 95-fold in the presence of omentum-tissue-conditioned media. More specifically, the presence of adipose tissue containing no milky spots revealed a 75% reduction in cancer cell migration, suggesting a key role of immune cells within the milky spots inducing a directed migration of these cells [35][21]. However, other cells, such as mesothelial cells and cancer-associated adipocytes, may also play a role [36][22].
In the early stage of tumour growth within the premalignant omentum, immune cells in the milky spots secrete protumourigenic factors, establishing a further crosstalk between tumour cells and local cancer-associated cells. This is further aided by hypoxia, resulting in the cancer cell migration, proliferation, and formation of secondary foci [35][21]. These micrometastatic proliferative tumour cells then aggregate and disrupt the structure of the milky spots, leaving immune cells dispersed within the tumour mass, suggesting that these resident immune cells in the milky spots aid tumour invasion and metastasis [37][23]. A study by Etzerodt et al. (2020) involving CD163 − and Tim4 − expressing macrophages showed the promotion of malignant ovarian cancer progression in the omentum via macrophage and tumour cell association [1]. For instance, the depletion of CD163 + Tim4 + macrophages prevented metastatic disease development and reduced ascite formation [1]. The secretion of CCL6 and CCL23 by omental macrophages significantly increased the migration and colonisation of EOC cells via binding to CCR1 receptors on cancer cells and inducing the activation of the ERK1/2 and AKT pathways, where ERK activation is pivotal in cancer cell survival through the upregulation of antiapoptotic proteins and the inhibition of caspase activity [38,39][24][25]. For instance, a reduction in omental macrophages caused a diminished level of EOC cell colonisation in the omentum, as did the inhibition of CCL6, highlighting that these macrophages possess a key role in tumour progression [39][25].
It has been found that the number of milky spots in the omentum increases in the presence of inflammatory cues/stimuli. For example, intraperitoneal injection of lipid A, a component of bacterial polysaccharide, increased the number of macrophages in the omentum [40][26]. Additionally, the number and size of milky spots increased in response to intraperitoneal injection of polydextran particles or polyacrylamide beads [41,42][27][28]. Interestingly, the omentum produced a CXCL13-mediated response, which is produced by CD4 T cells, and supported T cell-dependent B cell responses, including isotype switching, somatic hypermutation, and limited affinity maturation [43,44][29][30]. Tumour-associated macrophages have also been suggested to influence T cells by altering their cytotoxic function and recruitment in a cancer microenvironment in aged mice (reviewed in [45][31]). Moreover, the omentum also recruited effector T cells and produced CD4 + and CD8 + T cell responses to peritoneal antigens, suggesting that the adaptive immune system is a key component in inflammatory conditions, such as a growing tumour [44][30].
3. Diabetes Mellitus-Associated T Cell Senescence
The induction of T cell senescence was reported several years ago, although its disease-associated roles are now becoming more evident. T cell senescence is characterised by a reduction in the total naïve T cell pool, being replaced with differentiated T cells, such as effector memory (EM) T cells and terminally differentiated effector memory (EMRA) T cells [5,46][5][32]. The age and status of T cells are tracked by their cell-surface markers, as they do not express the costimulatory molecules CD27 and CD28, but do express CD57 and KLRG1, both of which function to reduce the proliferative capacity [46][32]. Senescent CD8 + T cells have recently been shown to increase in abundance in older donors with a twofold increase compared with younger donors in their early 20s, suggesting their major contribution to a reduced immune function and enhanced susceptibility to disease [47][33].
Metabolic disorders, such as obesity and T2DM, have also been shown to induce cellular senescence through an accumulation of these cells in organs as well as in the circulation [48][34]. For instance, Lau et al. reported an increase in EM and EMRA CD45RA re-expressing T cells in T2DM patients [11]. Overall, the authors showed that T2DM acted as a driving factor in the premature ageing of both CD4 + and CD8 + cells, increasing T cell differentiation and senescence, which contributed to systemic inflammation, as detected by raised levels of neutrophils [11]. Moreover, these senescent T cells expressed significantly higher numbers of the CX3CR1 chemokine receptor, indicating a promigratory phenotype that is seen during the early stages of tumour progression [11,49,50][11][35][36].
4. Could Premature Immunosenescence Contribute to EOC Progression in DM?
It was recently reported that T cell senescence appears in prediabetics, with increased expression of proinflammatory cytokines (TNF-α and IL-6) and cytotoxic enzymes (granzyme B) in CD28 − CD57 + CD8 + T cells [59][37]. Additionally, the levels of TNF-α and perforin were significantly increased in prediabetic patients, despite no increases in the CD4 + T cell number being observed in prediabetics compared with control subjects [59][37]. This indicates that the accumulation of senescent T cells can occur earlier in life, resulting in a more chronic proinflammatory environment. Moreover, premature ageing of T cells has been reported in T2DM and prediabetic patients [11,59][11][37]. This may indicate that in EOC patients, where age and T2DM are detrimental factors, early appearance and accumulation of immune senescence may contribute to a low-grade chronic inflammation in the peritoneum [102][38], specifically within and surrounding the milky spots. This could influence the formation of a premetastatic niche and lead to a poor prognosis of the disease. Therefore, further investigation is required to identify novel biomarkers for stratifying early-stage ovarian cancer patients suffering from T2DM [103][39] and to strategise and manage preventative measures, as early diagnosis in EOC patients is rare.
Nevertheless, research needs to be conducted to fully establish the link between senescent T cells in age, age-related diseases, chronic infections, and their role within the growth and metastasis of EOCs. Since early diagnosis of EOC is rare with a high incidence rate, and its risk is associated with senescence-inducing metabolic disorders, such as T2DM, efforts should be in place to detect immunosenescence earlier in life. Early diagnosis could lead to preventative therapeutic interventions. Currently, senolytic medications are known to selectively clear senescent cells and have been shown to both increase life span and alleviate age-related cardiovascular disease in mice [104,105][40][41]. Senolytics have also been demonstrated to decrease the population of senescent cells in human trials, while decreasing the numbers of SASP factors found within the blood [105,106][41][42]. Moreover, chimeric antigen receptor (CAR) T cells have been developed as a senolytic agent, which could identify markers and selectively target and destroy senescent cells [107][43]. This could be developed further to target to kill senescent T cells or to reverse exhaustion to switch CD8 + T cells to an antitumourigenic state [108][44]. Moreover, metformin, an antidiabetic drug, has shown some promises in alleviating cellular senescence and demonstrated preclinical anticancer, antiangiogenic effects in ovarian cancer; however, it lacks clinical evidence as an anticancer agent in ovarian cancer patients [109,110][45][46]. Thus, there is a possibility to clear senescent immune cells through an individual or a combination of treatment strategies within the milky spots and the omentum in disease states, such as T2DM and chronic infection. Further research is required to overcome barriers posed by solid tumours and their responses to immune surveillance and treatments, including immunotherapies [111][47].
References
- Etzerodt, A.; Moulin, M.; Doktor, T.K.; Delfini, M.; Mossadegh-Keller, N.; Bajenoff, M.; Sieweke, M.; Moestrup, S.K.; Auphan-Anezin, N.; Lawrence, T. Tissue-resident macrophages in omentum promote metastatic spread of ovarian cancer. J. Exp. Med. 2020, 217, e20191869.
- Harper, E.I.; Sheedy, E.F.; Stack, M.S. With Great Age Comes Great Metastatic Ability: Ovarian Cancer and the Appeal of the Aging Peritoneal Microenvironment. Cancers 2018, 10, 230.
- Liu, J.; Geng, X.; Li, Y. Milky spots: Omental functional units and hotbeds for peritoneal cancer metastasis. Tumor Biol. 2016, 37, 5715–5726.
- Frasca, D.; Blomberg, B.B. Adipose Tissue: A Tertiary Lymphoid Organ: Does It Change with Age? Gerontol. 2019, 66, 114–121.
- Callender, L.; Carroll, E.C.; Beal, R.W.J.; Chambers, E.; Nourshargh, S.; Akbar, A.; Henson, S.M. Human CD8+ EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell 2017, 17, e12675.
- Bakhru, A.; Buckanovich, R.J.; Griggs, J.J. The impact of diabetes on survival in women with ovarian cancer. Gynecol. Oncol. 2011, 121, 106–111.
- Shah, M.M.; Erickson, B.; Matin, T.; McGwin, G.; Martin, J.Y.; Daily, L.B.; Pasko, D.; Haygood, C.W.; Fauci, J.M.; Leath, C.A. Diabetes mellitus and ovarian cancer: More complex than just increasing risk. Gynecol. Oncol. 2014, 135, 273–277.
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832.
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522.
- Xia, C.; Rao, X.; Zhong, J. Role of T Lymphocytes in Type 2 Diabetes and Diabetes-Associated Inflammation. J. Diabetes Res. 2017, 2017, 1–6.
- Lau, E.; Carroll, E.C.; Callender, L.A.; Hood, G.A.; Berryman, V.; Pattrick, M.; Finer, S.; Hitman, G.A.; Ackland, G.L.; Henson, S.M. Type 2 diabetes is associated with the accumulation of senescent T cells. Clin. Exp. Immunol. 2019, 197, 205–213.
- Craig, E.R.; Londoño, A.I.; Norian, L.A.; Arend, R.C. Metabolic risk factors and mechanisms of disease in epithelial ovarian cancer: A review. Gynecol. Oncol. 2016, 143, 674–683.
- Nagle, C.M.; Dixon, S.C.; Jensen, A.; Kjaer, S.K.; Modugno, F.; DeFazio, A.; Fereday, S.; Hung, J.; Johnatty, S.E.; Australian Ovarian Cancer Study Group; et al. Obesity and survival among women with ovarian cancer: Results from the Ovarian Cancer Association Consortium. Br. J. Cancer 2015, 113, 817–826.
- Ignacio, R.M.C.; Lee, E.-S.; Wilson, A.J.; Beeghly-Fadiel, A.; Whalen, M.M.; Son, D.-S. Obesity-Induced Peritoneal Dissemination of Ovarian Cancer and Dominant Recruitment of Macrophages in Ascites. Immune Netw. 2018, 18, e47.
- Shirakawa, K.; Yan, X.; Shinmura, K.; Endo, J.; Kataoka, M.; Katsumata, Y.; Yamamoto, T.; Anzai, A.; Isobe, S.; Yoshida, N.; et al. Obesity accelerates T cell senescence in murine visceral adipose tissue. J. Clin. Investig. 2016, 126, 4626–4639.
- Conley, S.M.; Hickson, L.J.; Kellogg, T.A.; McKenzie, T.; Heimbach, J.K.; Taner, T.; Tang, H.; Jordan, K.L.; Saadiq, I.M.; Woollard, J.R.; et al. Human Obesity Induces Dysfunction and Early Senescence in Adipose Tissue-Derived Mesenchymal Stromal/Stem Cells. Front. Cell Dev. Biol. 2020, 8, 197.
- Mantovani, A.; Savino, B.; Locati, M.; Zammataro, L.; Allavena, P.; Bonecchi, R. The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev. 2010, 21, 27–39.
- Son, D.-S.; Kabir, S.M.; Dong, Y.-L.; Lee, E.; Adunyah, S.E. Inhibitory Effect of Tumor Suppressor p53 on Proinflammatory Chemokine Expression in Ovarian Cancer Cells by Reducing Proteasomal Degradation of IκB. PLoS ONE 2012, 7, e51116.
- Zhang, J.; He, T.; Xue, L.; Guo, H. Senescent T cells: A potential biomarker and target for cancer therapy. EBioMedicine 2021, 68, 103409.
- Cruz-Migoni, S.; Caamaño, J. Fat-Associated Lymphoid Clusters in Inflammation and Immunity. Front. Immunol. 2016, 7, 612.
- Clark, R.; Krishnan, V.; Schoof, M.; Rodriguez, I.; Theriault, B.; Chekmareva, M.; Rinker-Schaeffer, C. Milky Spots Promote Ovarian Cancer Metastatic Colonization of Peritoneal Adipose in Experimental Models. Am. J. Pathol. 2013, 183, 576–591.
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer-Associated Adipocytes Exhibit an Activated Phenotype and Contribute to Breast Cancer Invasion. Cancer Res. 2011, 71, 2455–2465.
- Gerber, S.A.; Rybalko, V.Y.; Bigelow, C.E.; Lugade, A.A.; Foster, T.; Frelinger, J.G.; Lord, E.M. Preferential Attachment of Peritoneal Tumor Metastases to Omental Immune Aggregates and Possible Role of a Unique Vascular Microenvironment in Metastatic Survival and Growth. Am. J. Pathol. 2006, 169, 1739–1752.
- Boussios, S.; Karathanasi, A.; Cooke, D.; Neille, C.; Sadauskaite, A.; Moschetta, M.; Zakynthinakis-Kyriakou, N.; Pavlidis, N.; Kyriakou, Z. PARP Inhibitors in Ovarian Cancer: The Route to "Ithaca". Diagnostics 2019, 9, 55.
- Krishnan, V.; Tallapragada, S.; Schaar, B.; Kamat, K.; Chanana, A.M.; Zhang, Y.; Patel, S.; Parkash, V.; Rinker-Schaeffer, C.; Folkins, A.K.; et al. Omental macrophages secrete chemokine ligands that promote ovarian cancer colonization of the omentum via CCR1. Commun. Biol. 2020, 3, 1–13.
- Ha, S.-A.; Tsuji, M.; Suzuki, K.; Meek, B.; Yasuda, N.; Kaisho, T.; Fagarasan, S. Regulation of B1 cell migration by signals through Toll-like receptors. J. Exp. Med. 2006, 203, 2541–2550.
- Shah, S.; Lowery, E.; Braun, R.K.; Martín, A.; Huang, N.; Medina, M.; Sethupathi, P.; Seki, Y.; Takami, M.; Byrne, K.; et al. Cellular Basis of Tissue Regeneration by Omentum. PLoS ONE 2012, 7, e38368.
- Litbarg, N.O.; Gudehithlu, K.P.; Sethupathi, P.; Arruda, J.A.L.; Dunea, G.; Singh, A.K. Activated omentum becomes rich in factors that promote healing and tissue regeneration. Cell Tissue Res. 2007, 328, 487–497.
- Kobayashi, S.; Murata, K.; Shibuya, H.; Morita, M.; Ishikawa, M.; Furu, M.; Ito, H.; Ito, J.; Matsuda, S.; Watanabe, T.; et al. A Distinct Human CD4+ T Cell Subset That Secretes CXCL13 in Rheumatoid Synovium. Arthritis Rheum. 2013, 65, 3063–3072.
- Rangel-Moreno, J.; Moyron-Quiroz, J.E.; Carragher, D.; Kusser, K.; Hartson, L.; Moquin, A.; Randall, T.D. Omental Milky Spots Develop in the Absence of Lymphoid Tissue-Inducer Cells and Support B and T Cell Responses to Peritoneal Antigens. Immunity 2009, 30, 731–743.
- Duong, L.; Radley, H.; Lee, B.; Dye, D.; Pixley, F.; Grounds, M.D.; Nelson, D.; Jackaman, C. Macrophage function in the elderly and impact on injury repair and cancer. Immun. Ageing 2021, 18, 1–11.
- Xu, W.; Larbi, A. Markers of T Cell Senescence in Humans. Int. J. Mol. Sci. 2017, 18, 1742.
- Martínez-Zamudio, R.I.; Dewald, H.K.; Vasilopoulos, T.; Gittens-Williams, L.; Fitzgerald-Bocarsly, P.; Herbig, U. Conclusive Identification of Senescent T Cells Reveals Their Abundance in Aging Humans. bioRxiv 2020.
- Macaulay, R.; Akbar, A.; Henson, S.M. The role of the T cell in age-related inflammation. AGE 2012, 35, 563–572.
- Slaney, C.Y.; Kershaw, M.; Darcy, P.K. Trafficking of T Cells into Tumors. Cancer Res. 2014, 74, 7168–7174.
- Saleh, R.; Nair, V.S.; Toor, S.M.; Taha, R.Z.; Murshed, K.; Al-Dhaheri, M.; Khawar, M.; Petkar, M.A.; Abu Nada, M.; Al-Ejeh, F.; et al. Differential gene expression of tumor-infiltrating CD8+ T cells in advanced versus early-stage colorectal cancer and identification of a gene signature of poor prognosis. J. Immunother. Cancer 2020, 8, e001294.
- Yi, H.-S.; Kim, S.Y.; Kim, J.T.; Lee, Y.-S.; Moon, J.S.; Kim, M.; Kang, Y.E.; Joung, K.H.; Lee, J.H.; Kim, H.J.; et al. T-cell senescence contributes to abnormal glucose homeostasis in humans and mice. Cell Death Dis. 2019, 10, 1–15.
- Carlow, D.A.; Gold, M.; Ziltener, H.J. Lymphocytes in the Peritoneum Home to the Omentum and Are Activated by Resident Dendritic Cells. J. Immunol. 2009, 183, 1155–1165.
- Arend, R.; Martinez, A.; Szul, T.; Birrer, M.J. Biomarkers in ovarian cancer: To be or not to be. Cancer 2019, 125, 4563–4572.
- Roos, C.M.; Zhang, B.; Palmer, A.; Ogrodnik, M.; Pirtskhalava, T.; Thalji, N.M.; Hagler, M.; Jurk, D.; Smith, L.A.; Casaclang-Verzosa, G.; et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 2016, 15, 973–977.
- Hickson, L.; Prata, L.L.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456.
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658.
- Amor, C.; Feucht, J.; Leibold, J.; Ho, Y.-J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T.; et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nat. Cell Biol. 2020, 583, 127–132.
- Sears, J.D.; Waldron, K.J.; Wei, J.; Chang, C. Targeting metabolism to reverse T-cell exhaustion in chronic viral infections. Immunology 2020, 162, 135–144.
- Urpilainen, E.; Puistola, U.; Boussios, S.; Karihtala, P. Metformin and ovarian cancer: The evidence. Ann. Transl. Med. 2020, 8, 1711.
- Fang, J.; Yang, J.; Wu, X.; Zhang, G.; Li, T.; Wang, X.; Zhang, H.; Wang, C.-C.; Liu, G.-H.; Wang, L. Metformin alleviates human cellular aging by upregulating the endoplasmic reticulum glutathione peroxidase 7. Aging Cell 2018, 17, e12765.
- Alard, E.; Butnariu, A.-B.; Grillo, M.; Kirkham, C.; Zinovkin, D.A.; Newnham, L.; Macciochi, J.; Pranjol, Z.I. Advances in Anti-Cancer Immunotherapy: Car-T Cell, Checkpoint Inhibitors, Dendritic Cell Vaccines, and Oncolytic Viruses, and Emerging Cellular and Molecular Targets. Cancers 2020, 12, 1826.
