As mentioned above, in preclinical models the consumption of diets with high AGE content (i.e., thermally processed or enriched with fructose) leads to the accumulation of AGEs within tissues with a high ECM content, such as intervertebral discs. Compared to low AGE diets, the AGE content of ECM located in intervertebral discs in mice fed with high AGE content diets increased by about 150 percent
[53][68]. From a structural point of view, one of the mechanisms of AGEs in altering tissue structure and function is the action of crosslink proteins. For instance, pentosidine is a crosslink between lysine and arginine residues belonging to the same or to different protein molecules
[54][69]. In tissues such as articular cartilage, stiffness and brittleness are associated to crosslinks of long-living proteins, such as aggrecan and collagen
[55][70], both present in bone and other ECM rich tissues
[56][71]. In these organs, AGE collagen crosslinks accumulate, implying a loss of ECM integrity and advancing the pathogenesis of the degenerative process
[57][72]. In addition, in vitro ECM glycation specifically increases tissue rigidity and brittleness
[58][73]. Collagen damage can be caused by increased crosslinking due to glycation
[59][60][61][74,75,76] resulting in structural disorder of the collagen fibrillar array. Thus, glycation has been shown to disrupt the organization of collagen in several ways: through increased fibril packing and diameter and by altering fibril organization
[62][77]; AGE adducts can twist and distort the collagen fibrillar array
[61][76]; and AGEs can alter molecular packing by increasing the distances between collagen chains and molecules
[63][78]. Indeed, in preclinical models the data indicate that biomechanical changes are partly driven by AGE crosslinking in the extracellular matrix, although it cannot be discounted that diet-derived AGES or AGE precursors could impinge oxidative modifications that result in damage to collagen, which is likely to increase the risk of mechanical injury in older mice or under higher load conditions
[53][68]. It has been suggested that crosslinking with AGE can lead to mechanically induced collagen triple-helical structure damage derived from accelerated enzyme degradation, transmitted through the intermolecular AGE crosslinks
[64][79]. The contribution of AGEs also goes further than the biomechanical changes induced in ECM, comprising changes in cellular responsese toi AGE-modified ECM, in particular the generation of a fibrosis-prone gene expression profile, which is widely documented in many complications of diabetes
[65][66][80,81]. Thus, AGEs induce enzymatic activity and catabolism of the matrix, mediated by binding to the AGE receptor (RAGE)
[67][82]. Previously data have suggested an important role for the AGE/RAGE pathway
[40][55] in DM and AGE-induced ECM degeneration, but there is still scope to further investigate the role of this interaction, particularly in relation to dietary AGEs in humans (see next section in this chapter). Moreover, it has been described that dAGEs do not always bind to RAGE
[68][83] so other RAGE-independent mechanisms can also operate. Some dAGEs, and particularly CML, are stable, chemically unreactive compounds that cannot damage the tissue by direct interaction with tissue proteins. Apart from CML, other Maillard reaction-generated products, such as dicarbonyl compounds (e.g., glyoxal, methylglyoxal and 3-deoxyglucosone), are also present in diets with high AGE contents. These reactive compounds could perform a direct modification of ECM
[69][84]. Nonetheless, there is controversy on whether these reactive compounds could contribute to AGE burden, since other researchers have shown that dietary dicarbonyls are unlikely to directly contribute to the endogenous pool of these compounds
[70][85]. It has also been suggested that high-fat diets induce in vivo dicarbonyl formation
[70][71][85,86]. Therefore, the increased level of endogenous AGEs observed in in vivo studies offering a roasted, high-fat diet might be partially explained by the high-fat induced formation of dicarbonyl compounds.
Further indications regarding dAGE health effects could be provided by studies in tissue distribution and renal clearance of labeled dAGEs. Rats supplied with AGEs derived from bread crust
[72][73][87,88] exhibit increased content of CML in tissues rich in ECM, such as tail tendon and heart. Similarly, mice supplied with CML-enriched diets also exhibit enrichment of aorta CML concentration
[74][89]. In these later experiments, in comparison with the control diet, the CML-enriched diet led to reduced endothelium-dependent relaxation, in association with increased aortic expression of RAGE and VCAM-1. This was strongly dependent on RAGE, since RAGE−/− mice were protected against these changes
[74][89].
Globally, dietary AGE content may be a crucial determinant of the accumulation of AGE crosslinks in tendons and for tissue compliance, relevant to the biological properties of ECM. Tessier et al.
[48][63] unequivocally identified a relationship between dietary CML and its deposition in organs using radio-labeled CML. However, there is no agreement to what degree the accumulation of dAGEs (and which AGE) in tissues could have pathological implications. In this sense, there is a high heterogeneity in serum AGE levels in healthy subjects, indicating that dAGEs might not necessarily be dangerous unless there is a concomitant disease. In this sense, patients with renal disease exhibiting an inefficient AGE excretion mechanism
[75][76][90,91] have a clear increase in circulating AGE levels. This rise is due in part to the impaired excretion of endogenous AGEs
[74][89] since a low dAGE diet intervention showed a marked decrease in circulating AGEs in these patients
[77][78][92,93]. Of note, skin autofluorescence, a purportedly indirect assessment of skin AGE, has been recently described as an independent risk factor for cardiovascular complications in individuals with uremia
[79][94].
It is noteworthy that very few papers discuss the potential influence of dAGEs in intracellular AGE modification in humans. Despite ample evidence in rodents
[80][95] even for neural tissue modification and validation studies in humans
[81][96], no exhaustive studies have been undertaken for the evaluation of dAGEs as a direct AGE modifier of intracellular proteins. These intracellular modifications
[82][83][97,98] play a key role in the processes where AGEs (endogenous) have been invoked. They may also play a role in the inflammatory status
[84][99]. However, the fact that dAGEs could enhance cellular oxidative stress and derived signaling, increasing in situ formation of AGE products or highly reactive AGE-precursors such as methylglyoxal
[85][100], gives a further dimension to the already intricate relationship of dAGEs with inflammation.
A very recent paper adds a novel layer of complexity in the dAGE relationship with inflammation. Snelson et al. have shown that long-term consumption of dAGEs leads to changes in intestinal barrier permeability in rodents
[86][101]. This alteration increases innate immune complement activation and local inflammation (especially evident at the renal level), via generation of the pro-inflammatory effector molecule complement 5a (C5a). These data reinforce previous evidence showing increased concentration of several cytokines, such as IL-16, IL-1α, ICAM, TIMP-1 and C5a, in mice fed with high AGE dietary contents
[87][102]. Mechanistically, in vitro data show that AGE-modified albumin cleaves C5 to generate C5a
[88][103]. In humans, lutein treatment induces decreased C5a concentrations in patients with early macular degeneration
[89][104]. Indeed, C5a tissue levels are enhanced in the vitreous of patients with proliferative diabetic retinopathy
[90][105]. However, whether changes in dAGEs impinge C5a variations in humans is still unknown. Of note, a decreased burden of dAGEs changes gut microbiome
[91][106], which is a primary driver in the dAGE-derived innate immune complement activation. Thus, it may be proposed that dAGEs acting either directly on ECM proteins, or indirectly altering intestinal permeability barriers, could change inflammatory status in humans.
2.2. Dietary AGEs and Receptor-Mediated Mechanisms
As mentioned previously, AGEs tend to accumulate in a wide range of chronic conditions, such as DM, vascular disease, arthritis, aging, cancer, and neurodegeneration
[92][93][94][95][107,108,109,110]. Apart from endogenous production, dietary AGEs are important contributors to the body’s AGE pool.
Many AGEs-binding proteins have been described so far, most of them involved in the endocytic uptake, breakdown, and removal of AGEs from the circulation
[96][111]. The exception is the protein known as RAGE, which is the central transduction receptor for AGEs. RAGE belongs to the immunoglobulin superfamily of molecules. Its structure comprises a multi-ligand binding extracellular domain, a membrane-spanning domain, and an intracellular carboxyl-terminal domain
[97][112]. The extracellular domain is composed of three smaller domains, one V-type domain with homology to immunoglobulin variable domains, and two C-type domains with homology to the immunoglobulin constant domains
[98][113]. While RAGE is the product of a single gene, alternative splicing generates soluble RAGE, lacking both the membrane-spanning and intracellular domains
[99][100][114,115]. Additionally, extracellular metalloproteinases can cleave cell surface RAGE leading to an additional soluble receptor form. Independently of the mechanisms involved, soluble RAGE molecules serve as decoys for circulating AGEs and other ligands
[101][116].
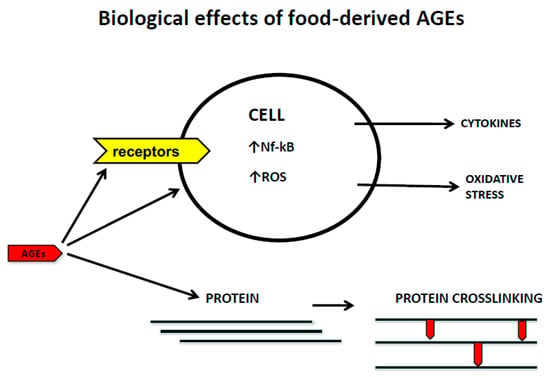
The recognition of AGEs by RAGE initiates complex signaling pathways, including reactive oxygen species (ROS), p21ras, erk1/2 (p44/p42) MAP kinases, p38 and SAPK/JNK MAP kinases, rhoGTPases, phosphoinositol-3 kinase and the JAK/STAT pathways, rendering crucial down-stream inflammatory consequences, such as the activation of crucial transcription factors such as NF-κB, AP-1, and Stat-3. The activation of NFkB up-regulates RAGE expression itself, thus generating a positive feed-forward loop rendering more inflammation
[102][117].
At present, the binding capacity of RAGE has been extended to a diverse series of other ligands beyond AGEs, many of which can be defined as pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), leading to the recognition that RAGE is another pattern recognition receptor (PRR). This ligand binding diversity adds more complexity to the intracellular signaling network of RAGE, considering that this receptor shares ligands and pathways with toll-like receptors, particularly with TLR-2 and -4
[103][118].
The short cytoplasmic tail lacks intrinsic kinase activity, however, compelling data derived from both in vitro and in vivo experiments indicate that the cytoplasmic domain is essential for intracellular signaling, by recruiting adaptor proteins, such as Diaphanous-1 and toll-interleukin 1 receptor domain-containing adaptor protein (TIRAP), or the extracellular signal-regulated kinase Erk1
[104][105][106][119,120,121].
It is noteworthy that the complexity of signaling has been further evidenced by several reports showing RAGE interactions with other plasma membrane proteins, as occurs with the alternative adaptor molecule DAP10, which seems to be crucial for enhancing the RAGE-mediated signaling, as well as the interaction of RAGE with chemotactic G-protein-coupled receptors (GPCRs) on the plasma membrane
[107][108][122,123]. Furthermore, a cognate ligand-independent mechanism for RAGE transactivation has been recently reported to occur following activation of the AT1R in different cell types
[109][124].
Additionally, RAGE oligomerization has been highlighted as a crucial event in signaling
[110][125] because disruption of the self-association results in reduced mitogen-activated protein kinase phosphorylation and nuclear factor NF-κB activation
[111][126].
At present, a huge body of compelling evidence demonstrates RAGE and its ligands are a major inflammatory driving mechanism in the pathogenesis of many clinical entities where inflammation is an important element in the onset and/or progression of the disease
[109][110][111][112][113][114][124,125,126,127,128,129].
Most of the AGE pathological actions are due to the interaction with the full-RAGE anchorage at the cytoplasmic membrane. However, one interesting point is raised by some reports showing the presence of intracellular AGEs, particularly CML and MGO
[18][115][18,130]. These findings are particularly important because RAGE has also been reported to be located intracellularly and co-localized with endosomes, particularly in T cells from patients with T1DM
[116][131]. Thus, it is tempting to speculate that intracellular AGEs may represent a “danger signal”, which may be then recognized by endosomal RAGE and thus activating intracellular signaling pathways, as occurs with other pattern recognition receptors
[117][132].
In recent years, NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) has emerged as a crucial intracellular sensor that detects a broad range of microbial motifs, endogenous danger signals, and environmental irritants, resulting in the formation and activation of the NLRP3 inflammasome
[118][133]. Assembly of the NLRP3 inflammasome leads to caspase 1-dependent release of the pro-inflammatory cytokines IL-1β and IL-18, as well as to gasdermin D-mediated pyroptotic cell death
[119][134]. The role of AGES in the activation of NLRP3 inflammasome remains controversial. While some reports support the role of AGEs in the activation of the NLRP3 inflammasome in endothelial cells
[120][135], placental tissue
[121][136], chondrocytes
[122][137], and podocytes
[123][138], other authors have demonstrated that AGEs could impair NLRP3 inflammasome activation
[124][125][139,140]. Thus, AGEs regulation of NLRP3 inflammasome activity appears to be highly dependent on cell type, and this issue, therefore, requires further investigation. Furthermore, the primary inflammasome research interests have been focused on NLRP3, and the effects of AGEs on the subset of NLRs other than NLRP3 is still a matter of research.