Protein calories consumed by people all over the world approximate 15–20% of their energy intake. This makes protein a major nutritional imperative. Today, we are facing an unprecedented challenge to produce and distribute adequate protein to feed over nine billion people by 2050, in an environmentally sustainable and affordable way. Plant-based proteins present a promising solution to our nutritional needs due to their long history of crop use and cultivation, lower cost of production, and easy access in many parts of the world. However, plant proteins have comparatively poor functionality, defined as poor solubility, foaming, emulsifying, and gelling properties, limiting their use in food products. Relative to animal proteins, including dairy products, plant protein technology is still in its infancy. To bridge this gap, advances in plant protein ingredient development and the knowledge to construct plant-based foods are sorely needed.
- plant proteins
- future foods
- animal alternatives
- nutrition
1. Introduction
23. Creating Future Foods Using Plant Proteins
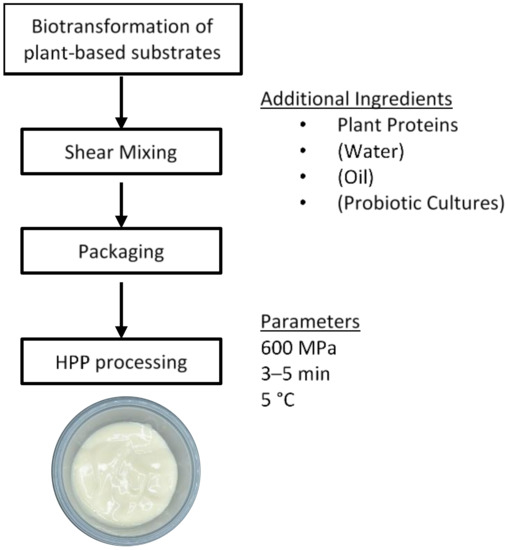
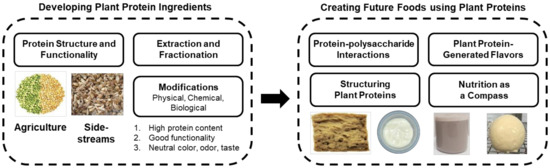
The previous sections have described the gaps in plant protein ingredient science and technology. However, we usually eat foods, and not individual ingredients. After obtaining highly functional plant proteins, the challenge is to transform these ingredients into delicious and nutritious foods. The following sections describe some important factors: the role of protein–polysaccharide interactions, the ability to structure plant proteins into fibers and gels, the inclusion of flavors derived from plant proteins, and nutrition to guide the development of plant-based foods.23.1. Protein–Polysaccharide Interactions
32. Structuring Plant Proteins
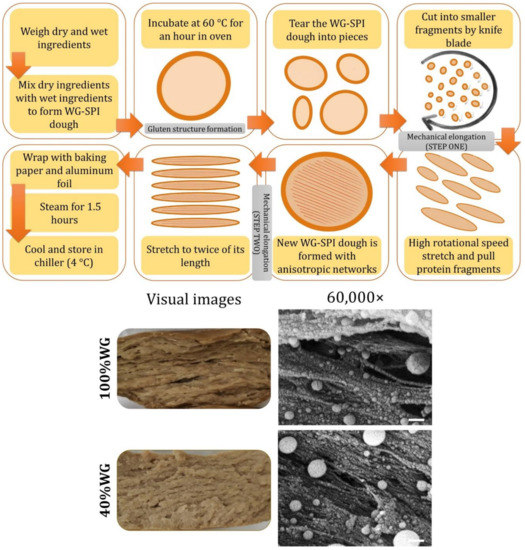
32.1. Formation of Fibrous Structures
32.2. Formation of Gels