Laser-Induced Breakdown Spectroscopy (LIBS) has been firstly introduced and proposed for analytical applications almost immediately after the invention of the laser in 1960. Since then, it has been proposed and today is widely used as an alternative analytical method for numerous applications. The operating principle of LIBS is quite simple and is based on the interaction of a powerful enough laser beam, focused usually on or in a sample, inducing a dielectric breakdown of the material, thus resulting in plasma formation consisting of excited and non-excited atoms and molecules, fragments of molecular species, electrons and ions, and emitting characteristic radiations, whose spectroscopic analysis can in principle provide the elemental composition fingerprint of the material. The required instrumentation consisting basically of a laser source, and a spectrometer/monochromator equipped with the appropriate light detector (nowadays being almost exclusively some CCD or ICCD type detector) is relatively simple and economically affordable, while significant progresses have been achieved to small size and/or portable equipment, facilitating largely the in situ operation.
- Laser-Induced Breakdown Spectroscopy
- LIBS
- food analysis
- spectroscopy
1. Overview
Laser-Induced Breakdown Spectroscopy (LIBS), having reached a level of maturity during the last few years, is generally considered as a very powerful and efficient analytical tool, and it has been proposed for a broad range of applications, extending from space exploration down to terrestrial applications, from cultural heritage to food science and security. Over the last decade, there has been a rapidly growing sub-field concerning the application of LIBS for food analysis, safety, and security, which along with the implementation of machine learning and chemometric algorithms opens new perspectives and possibilities.
2. Laser-Induced Breakdown Spectroscopy (LIBS)
3. LIBS principle of operation
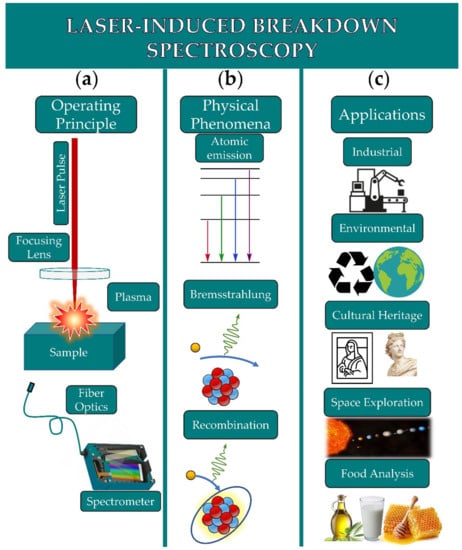
4. Conclusions
References
- Brech, F.; Cross, L.; Optical Microemission stimulated by a Ruby Maser. Appl. Spectrosc. 1962, 16, 59.
- Baudelet, M.; Smith, B.W.; The first years Of Laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2013, 28, 624.
- Miziolek, A.W.; Palleschi, V.; Schechter, I. . Laser induced breakdown spectroscopy; Cambridge University Press: Cambridge, UK, 2008; pp. 1-36.
- Syed Kifayat Hussain Shah; Javed Iqbal; Pervaiz Ahmad; Mayeen Uddin Khandaker; Sirajul Haq; Muhammad Naeem; Laser induced breakdown spectroscopy methods and applications: A comprehensive review. Radiation Physics and Chemistry 2019, 170, 108666, 10.1016/j.radphyschem.2019.108666.
- Giorgio S. Senesi; Russell S. Harmon; Richard R. Hark; Field-portable and handheld laser-induced breakdown spectroscopy: Historical review, current status and future prospects. Spectrochimica Acta Part B: Atomic Spectroscopy 2020, 175, 106013, 10.1016/j.sab.2020.106013.
- Reinhard Noll; Holger Bette; Adriane Brysch; Marc Kraushaar; Ingo Mönch; Laszlo Peter; Volker Sturm; Laser-induced breakdown spectrometry — applications for production control and quality assurance in the steel industry. Spectrochimica Acta Part B: Atomic Spectroscopy 2001, 56, 637-649, 10.1016/s0584-8547(01)00214-2.
- Rosalba Gaudiuso; Marcella Dell’Aglio; Olga De Pascale; Giorgio S. Senesi; Alessandro De Giacomo; Laser Induced Breakdown Spectroscopy for Elemental Analysis in Environmental, Cultural Heritage and Space Applications: A Review of Methods and Results. Sensors 2010, 10, 7434-7468, 10.3390/s100807434.
- Jean-Baptiste Sirven; Béatrice Sallé; Patrick Mauchien; Jean-Luc Lacour; Sylvestre Maurice; Gérard Manhes; Feasibility study of rock identification at the surface of Mars by remote laser-induced breakdown spectroscopy and three chemometric methods. Journal of Analytical Atomic Spectrometry 2007, 22, 1471-1480, 10.1039/b704868h.
- Yang-Ting Fu; Wei-Lun Gu; Zong-Yu Hou; Sher Afgan Muhammed; Tian-Qi Li; Yun Wang; Zhe Wang; Mechanism of signal uncertainty generation for laser-induced breakdown spectroscopy. Frontiers of Physics 2020, 16, 1-10, 10.1007/s11467-020-1006-0.
- Tomoko Takahashi; Blair Thornton; Quantitative methods for compensation of matrix effects and self-absorption in Laser Induced Breakdown Spectroscopy signals of solids. Spectrochimica Acta Part B: Atomic Spectroscopy 2017, 138, 31-42, 10.1016/j.sab.2017.09.010.
- Daniel Fernandes Andrade; Edenir Rodrigues Pereira-Filho; Dulasiri Amarasiriwardena; Current trends in laser-induced breakdown spectroscopy: a tutorial review. Applied Spectroscopy Reviews 2020, 56, 98-114, 10.1080/05704928.2020.1739063.
- Lu-Ning Li; Xiang-Feng Liu; Fan Yang; Wei-Ming Xu; Jian-Yu Wang; Rong Shu; A review of artificial neural network based chemometrics applied in laser-induced breakdown spectroscopy analysis. Spectrochimica Acta Part B: Atomic Spectroscopy 2021, 180, 106183, 10.1016/j.sab.2021.106183.
- Erik Képeš; Jakub Vrábel; Sára Střítežská; Pavel Pořízka; Jozef Kaiser; Benchmark classification dataset for laser-induced breakdown spectroscopy. Scientific Data 2020, 7, 1-4, 10.1038/s41597-020-0396-8.
- Jakub Vrábel; Erik Kepes; Ludovic Duponchel; Vincent Motto-Ros; Cécile Fabre; Sven Connemann; Frederik Schreckenberg; Paul Prasse; Daniel Riebe; Rajendhar Junjuri; et al.Manoj Kumar GundawarXiaofeng TanPavel PořízkaJozef Kaiser Classification of challenging Laser-Induced Breakdown Spectroscopy soil sample data - EMSLIBS contest. Spectrochimica Acta Part B: Atomic Spectroscopy 2020, 169, 105872, 10.1016/j.sab.2020.105872.
- Maria Markiewicz-Keszycka; Raquel Cama-Moncunill; Maria Pietat Casado-Gavalda; Carl Sullivan; Patrick J Cullen; Laser-induced breakdown spectroscopy for food authentication. Current Opinion in Food Science 2019, 28, 96-103, 10.1016/j.cofs.2019.10.002.
- Abrahan Velásquez-Ferrín; Diego Victor Babos; César Marina-Montes; Jesús Anzano; Rapidly growing trends in laser-induced breakdown spectroscopy for food analysis. Applied Spectroscopy Reviews 2020, 56, 492-512, 10.1080/05704928.2020.1810060.
- M. Capitelli; Anna Rita Casavola; Gianpiero Colonna; Alessandro De Giacomo; Laser-induced plasma expansion: theoretical and experimental aspects. Spectrochimica Acta Part B: Atomic Spectroscopy 2004, 59, 271-289, 10.1016/j.sab.2003.12.017.
- Alessandro De Giacomo; Jörg Hermann; Laser-induced plasma emission: from atomic to molecular spectra. Journal of Physics D: Applied Physics 2017, 50, 183002, 10.1088/1361-6463/aa6585.
- Kramida, A.; Ralchenko, Y.; Reader, J.; NIST ASD Team. NIST At. Spectra Database (Version 5.8). . NIST Atomic Spectra Database. Retrieved 2021-8-27
- Lovas, F.J.; Tiemann, E.; Coursey, J.S.; Kotochigova, S.A.; Chang, J.; Olsen, K.; Dragoset, R.A. Diatomic Spectral Database (Version 2.1); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2005. . NIST Diatomic Spectral Database. Retrieved 2021-8-27
