The NADPH oxidase Nox4 is a hydrogen peroxide (H2O2)-producing enzyme, with the highest expression in the kidney. As the kidney is involved in volume and blood pressure control through sodium handling, we set out to determine the impact of a low sodium diet on these parameters in WT and Nox4-/- mice. Nox4 expression in the murine kidney was restricted to the proximal tubule. Nevertheless, low-sodium-induced weight loss and sodium sparing function was similar in WT and Nox4-/- mice, disputing an important function of renal Nox4 in sodium handling. In contrast, a low sodium diet resulted in a reduction in systolic blood pressure in Nox4-/- as compared to WT mice. This was associated with a selectively lower pressure to heart-rate ratio, as well as heart to body weight ratio. In general, a low sodium diet leads to activation of sympathetic tone and the renin angiotensin system, which subsequently increases peripheral resistance. Our observations suggest that the control by this system is attenuated in Nox4-/- mice, resulting in lower blood pressure in response to low sodium.
- NADPH oxidase 4
- proximal tubule cells
- reactive oxygen species
1. Introduction
2. Nox4 Expression Is Restricted to Proximal Tubule Cells
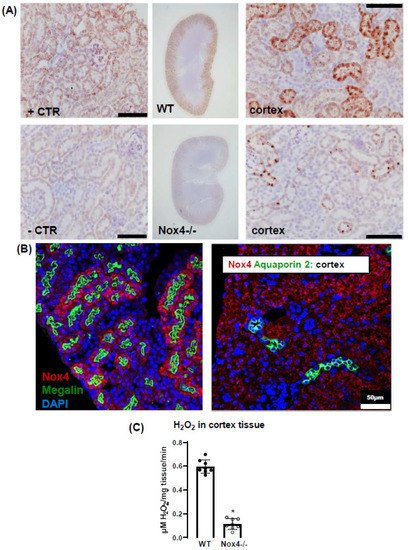
3. Nox4 Contributes to H2O2 Production of the Renal Cortex
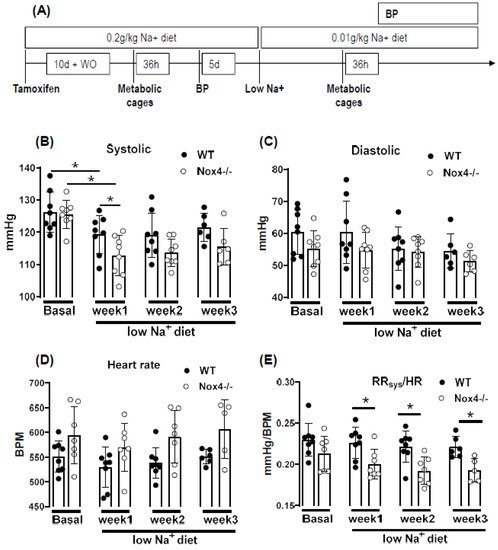
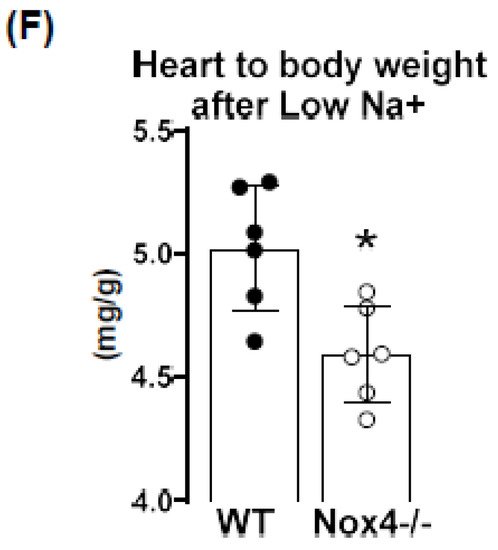
4. Knockout of Nox4 Lowers Blood Pressure and Cardiac Mass in Response to Low Sodium Diet
5. Discussion
In this study, we report that a low sodium diet results in an acute reduction in systolic blood pressure and a prolonged reduction in peripheral resistance in Nox4-/- mice. Despite the high expression of Nox4 in the kidney, this effect was unrelated to salt and water intake and renal sodium handling. Sodium restriction resulted in weight loss and renal sodium sparing, and this effect was similar between WT and Nox4-/- mice. Collectively, these data support the previous report of Nox4 in renal blood pressure control [27][25] but exclude a direct effect on sodium handling as an underlying mechanism. In the present study, we observe a high expression of Nox4 in the proximal renal tubule. Our data are in line with the single-cell sequencing atlas of the murine kidney [28][26], where Nox4 is expressed in the cluster of proximal tubule cells and a novel cluster that is also positive for megalin. In addition, in the human kidney, Nox4 appears to localize to the proximal tubule [18]. Despite these data on expression, very little is known concerning the function of Nox4 in the kidney. The location in the proximal tubule makes studying the function of Nox4 difficult, given that the physiological function of these cells depends on directionality, which is challenging in model systems of isolated cultured cells. Our past data indicated that Nox4 expression in the kidney is highest under quiescent conditions in healthy animals, whereas inflammation and diseases such as diabetes decreased Nox4 [9]. The finding that renal disease reduces Nox4 expression has, meanwhile, been recapitulated by others [18,29][18][27] and suggests that Nox4 is a marker for healthy, differentiated, intact renal tissue. This behavior also has significance for renal cell culture models: the isolation of proximal tubule cells leads to a rapid loss in Nox4. Therefore, renal proximal tubule cell lines are alternatives. The opossum kidney OK cell line (from Didelphis virginiana) is a broadly used model to study ion transport and membrane trafficking mechanisms in the proximal tubule. Transcriptomics of these cells, compared with mammalian proximal tubule cells, however, reveal that Nox4 expression is also lost in this cultured cell line [30][28]. Therefore, the search for a function of Nox4 has to rely on in vivo data. Given that the transport processes in the proximal tubule are largely sodium-coupled, studying sodium handling and its indirect consequence, plasma volume and blood pressure, can be seen as first approaches to dissect the function of Nox4 in the kidney. The proximal tubule reabsorbs two-thirds of filtered Na+ [31][29] and, consequently, is essential in sodium homeostasis. It is also important to note that, despite this behavior, the contribution of this renal segment to volume- and blood-pressure-control is, at least, controversial. From the knowledge available to date, the transport processes in this renal segment are not well controlled, potentially with the exception of phosphate reabsorption, which is inhibited by parathormone. Tubular-glomerular feedback (TGF), which controls proximal tubular urine flux, has a sensor in the distal tubule and affects glomerular filtration rate. To date, TGF has not been linked to Nox4; comparatively, it is associated with nitric oxide and adenosine [32][30]. The second system that is relevant in conjunction with low sodium is the renin–angiotensin system (RAS) because a low sodium diet increases RAS activity by volume depletion and subsequent sympathetic nerve activation. The combination of increased sympathetic tone and RAS activation usually compensates for the effect of hypovolemia on blood pressure: cardiac output, renal water retention and peripheral resistance all increase. The fact that the blood pressure/heart rate ratio of Nox4 mice on a low sodium diet was significantly lower than that in wild-type mice, suggests that neither peripheral resistance nor volume retention can be adequately increased after deletion of Nox4. As body weight and sodium excretion were similar between WT and Nox4-/- mice, differences in peripheral resistance should be considered. Interestingly, cerebral knockdown of Nox4 resulted in attenuated sympathico-excitation in response to cardiac damage [33][31]. Whether Nox4 impacts directly on peripheral resistance is unclear. It has been suggested that SERCA is oxidized by Nox4 in the heart and endothelium [34,35][32][33] and also in TRP-channels [36[34][35][36][37],37,38,39], which contribute to the control of resting calcium, and are targets of Nox4. Moreover, Nox4 has been linked to smooth muscle cell differentiation and, thus, contractility [40,41,42,43][38][39][40][41]. Collectively, these observations provide support for a role of Nox4 in vascular tone control. Additional studies will be needed to substantiate this assumption. Is there any evidence for a direct function of reactive oxygen species for renal hypertension? Superoxide anions lead to reduced medullary blood flow and increased sodium retention and, thus, hypertension [7], as shown by in vivo treatment with a superoxide dismutase mimetic. Moreover, superoxide inhibits proximal tubule fluid reabsorption in spontaneously hypertensive rats [44][42]. Renal hemodynamic and excretory functions, such as urine flow, sodium excretion and glomerular filtration were increased in hypertensive rats infused with the superoxide scavenger tempol without altering arterial pressure [8]. In contrast, infusion of H2O2 directly into the renal medulla increases mean arterial pressure [6]. Nox4 has been linked to renal hypertension and sodium retention in Dahl salt-sensitive rats, where volume expansion is considered to be the main cause of salt-sensitive hypertension [45][43]. Our results corroborate the findings in the Dahl salt-sensitive rats; however, we cannot link the effect of Nox4 to Na+ homeostasis because Na+ excretion and clearance were similar in WT and Nox4-/- mice. On the other hand, the effects might have been so subtle and transient that our study was not sensitive enough to detect them. The current study has several limitations. Blood pressure was measured by tail cuff technology, which is less accurate than telemetry. Moreover, we only estimated cardiac output and peripheral resistance from the blood pressure to heart rate ratio. A true determination of cardiac output and peripheral resistance would have required indicator injection dilution methodology. Moreover, metabolic cages impose a considerable amount of stress on mice. Food and fluid intake in the cages is low, which is also documented in the present study by the substantial weight loss. A possible alternative would have been clearance measurements using radioactive isotopes, but this technology was not available to us.References
- Araujo, M.; Wilcox, C.S. Oxidative Stress in Hypertension: Role of the Kidney. Antioxidants Redox Signal. 2014, 20, 74–101.
- Gill, P.S.; Wilcox, C.S. NADPH Oxidases in the Kidney. Antioxidants Redox Signal. 2006, 8, 1597–1607.
- Chabrashvili, T.; Tojo, A.; Onozato, M.L.; Kitiyakara, C.; Quinn, M.; Fujita, T.; Welch, W.J.; Wilcox, C.S. Expression and Cellular Localization of Classic NADPH Oxidase Subunits in the Spontaneously Hypertensive Rat Kidney. Hypertension 2002, 39, 269–274.
- Sedeek, M.; Hébert, R.L.; Kennedy, C.R.; Burns, K.D.; Touyz, R.M. Molecular mechanisms of hypertension: Role of Nox family NADPH oxidases. Curr. Opin. Nephrol. Hypertens. 2009, 18, 122–127.
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.; De Lucca Camargo, L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539.
- Makino, A.; Skelton, M.M.; Zou, A.-P.; Cowley, A.W. Increased Renal Medullary H2O2 Leads to Hypertension. Hypertension 2003, 42, 25–30.
- Makino, A.; Skelton, M.M.; Zou, A.-P.; Roman, R.J.; Cowley, A.W. Increased Renal Medullary Oxidative Stress Produces Hypertension. Hypertension 2002, 39, 667–672.
- Kopkan, L.; Castillo, A.; Navar, L.G.; Majid, D.S.A. Enhanced superoxide generation modulates renal function in ANG II-induced hypertensive rats. Am. J. Physiol. Physiol. 2006, 290, F80–F86.
- Babelova, A.; Avaniadi, D.; Jung, O.; Fork, C.; Beckmann, J.; Kosowski, J.; Weissmann, N.; Anilkumar, N.; Shah, A.; Schaefer, L.; et al. Role of Nox4 in murine models of kidney disease. Free. Radic. Biol. Med. 2012, 53, 842–853.
- Prior, K.-K.; Leisegang, M.S.; Josipovic, I.; Löwe, O.; Shah, A.M.; Weissmann, N.; Schröder, K.; Brandes, R.P. CRISPR/Cas9-mediated knockout of p22phox leads to loss of Nox1 and Nox4, but not Nox5 activity. Redox Biol. 2016, 9, 287–295.
- Takac, I.; Schröder, K.; Zhang, L.; Lardy, B.; Anilkumar, N.; Lambeth, J.D.; Shah, A.; Morel, F.; Brandes, R.P. The E-loop Is Involved in Hydrogen Peroxide Formation by the NADPH Oxidase Nox4. J. Biol. Chem. 2011, 286, 13304–13313.
- Löwe, O.; Rezende, F.; Heidler, J.; Wittig, I.; Helfinger, V.; Brandes, R.P.; Schröder, K. BIAM switch assay coupled to mass spectrometry identifies novel redox targets of NADPH oxidase 4. Redox Biol. 2019, 21, 101125.
- Santos, C.X.; Hafstad, A.D.; Beretta, M.; Zhang, M.; Molenaar, C.; Kopec, J.; Fotinou, D.; Murray, T.V.; Cobb, A.M.; Martin, D.; et al. Targeted redox inhibition of protein phosphatase 1 by Nox4 regulates eIF 2α-mediated stress signaling. EMBO J. 2016, 35, 319–334.
- Yang, Q.; Wu, F.-R.; Wang, J.-N.; Gao, L.; Jiang, L.; Li, H.-D.; Ma, Q.; Liu, X.-Q.; Wei, B.; Zhou, L.; et al. Nox4 in renal diseases: An update. Free Radic. Biol. Med. 2018, 124, 466–472.
- Holterman, C.; Read, N.C.; Kennedy, C.R.J. Nox and renal disease. Clin. Sci. 2014, 128, 465–481.
- Thallas-Bonke, V.; Jandeleit-Dahm, K.A.; Cooper, M.E. Nox-4 and progressive kidney disease. Curr. Opin. Nephrol. Hypertens. 2015, 24, 74–80.
- Liang, Y.; Liu, H.; Fang, Y.; Lin, P.; Lu, Z.; Zhang, P.; Jiao, X.; Teng, J.; Ding, X.; Dai, Y. Salvianolate ameliorates oxidative stress and podocyte injury through modulation of NOX4 activity in db/db mice. J. Cell. Mol. Med. 2021, 25, 1012–1023.
- Rajaram, R.D.; Dissard, R.; Faivre, A.; Ino, F.; Delitsikou, V.; Jaquet, V.; Cagarelli, T.; Lindenmeyer, M.; Jansen-Duerr, P.; Cohen, C.; et al. Tubular NOX4 expression decreases in chronic kidney disease but does not modify fibrosis evolution. Redox Biol. 2019, 26, 101234.
- Shi, Q.; Lee, D.-Y.; Féliers, D.; Abboud, H.E.; Bhat, M.A.; Gorin, Y. Interplay between RNA-binding protein HuR and Nox4 as a novel therapeutic target in diabetic kidney disease. Mol. Metab. 2020, 36, 100968.
- Jha, J.C.; Thallas-Bonke, V.; Banal, C.; Gray, S.P.; Chow, B.S.M.; Ramm, G.; Quaggin, S.E.; Cooper, M.E.; Schmidt, H.H.H.W.; Jandeleit-Dahm, K.A. Podocyte-specific Nox4 deletion affords renoprotection in a mouse model of diabetic nephropathy. Diabetologia 2016, 59, 379–389.
- You, Y.-H.; Quach, T.; Saito, R.; Pham, J.; Sharma, K. Metabolomics Reveals a Key Role for Fumarate in Mediating the Effects of NADPH Oxidase 4 in Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2015, 27, 466–481.
- Thallas-Bonke, V.; Tan, S.M.; Lindblom, R.S.; Snelson, M.; Granata, C.; Jha, J.C.; Sourris, K.C.; Laskowski, A.; Watson, A.; Tauc, M.; et al. Targeted deletion of nicotinamide adenine dinucleotide phosphate oxidase 4 from proximal tubules is dispensable for diabetic kidney disease development. Nephrol. Dial. Transplant. 2021, 36, 988–997.
- Cowley, A.W.; Abe, M.; Mori, T.; O’Connor, P.M.; Ohsaki, Y.; Zheleznova, N.N. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and hypertension. Am. J. Physiol. Physiol. 2015, 308, F179–F197.
- Serrander, L.; Cartier, L.; Bedard, K.; Banfi, B.; Lardy, B.; Plastre, O.; Sienkiewicz, A.; Fórró, L.; Schlegel, W.; Krause, K.-H. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem. J. 2007, 406, 105–114.
- Cowley, J.A.W.; Yang, C.; Zheleznova, N.N.; Staruschenko, A.; Kurth, T.; Rein, L.; Kumar, V.; Sadovnikov, K.; Dayton, A.; Hoffman, M.; et al. Evidence of the Importance of Nox4 in Production of Hypertension in Dahl Salt-Sensitive Rats. Hypertension 2016, 67, 440–450.
- Park, J.; Shrestha, R.; Qiu, C.; Kondo, A.; Huang, S.; Werth, M.; Li, M.; Barasch, J.; Suszták, K. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 2018, 360, 758–763.
- Khodo, S.N.; Dizin, E.; Sossauer, G.; Szanto, I.; Martin, P.-Y.; Feraille, E.; Krause, K.H.; De Seigneux, S. NADPH-Oxidase 4 Protects against Kidney Fibrosis during Chronic Renal Injury. J. Am. Soc. Nephrol. 2012, 23, 1967–1976.
- Eshbach, M.L.; Sethi, R.; Avula, R.; Lamb, J.; Hollingshead, D.J.; Finegold, D.N.; Locker, J.D.; Chandran, U.R.; Weisz, O.A. The transcriptome of the Didelphis virginiana opossum kidney OK proximal tubule cell line. Am. J. Physiol. Physiol. 2017, 313, F585–F595.
- McDonough, A.A. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am. J. Physiol. Integr. Comp. Physiol. 2010, 298, R851–R861.
- Satriano, J.; Wead, L.; Cardús, A.; Deng, A.; Boss, G.R.; Thomson, S.C.; Blantz, R.C. Regulation of ecto-5′-nucleotidase by NaCl and nitric oxide: Potential roles in tubuloglomerular feedback and adaptation. Am. J. Physiol. Physiol. 2006, 291, F1078–F1082.
- Infanger, D.W.; Cao, X.; Butler, S.D.; Burmeister, M.A.; Zhou, Y.; Stupinski, J.A.; Sharma, R.V.; Davisson, R.L. Silencing Nox4 in the Paraventricular Nucleus Improves Myocardial Infarction–Induced Cardiac Dysfunction by Attenuating Sympathoexcitation and Periinfarct Apoptosis. Circ. Res. 2010, 106, 1763–1774.
- Sun, Q.-A.; Hess, D.T.; Nogueira, L.; Yong, S.; Bowles, D.E.; Eu, J.; Laurita, K.R.; Meissner, G.; Stamler, J.S. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. Proc. Natl. Acad. Sci. USA 2011, 108, 16098–16103.
- Evangelista, A.M.; Thompson, M.D.; Bolotina, V.M.; Tong, X.; Cohen, R.A. Nox4- and Nox2-dependent oxidant production is required for VEGF-induced SERCA cysteine-674 S-glutathiolation and endothelial cell migration. Free Radic. Biol. Med. 2012, 53, 2327–2334.
- Ding, R.; Jiang, H.; Sun, B.; Wu, X.; Li, W.; Zhu, S.; Liao, C.; Zhong, Z.; Chen, J. Advanced oxidation protein products sensitized the transient receptor potential vanilloid 1 via NADPH oxidase 1 and 4 to cause mechanical hyperalgesia. Redox Biol. 2016, 10, 1–11.
- Lin, C.-S.; Lee, S.-H.; Huang, H.-S.; Chen, Y.-S.; Ma, M.-C. H2O2 generated by NADPH oxidase 4 contributes to transient receptor potential vanilloid 1 channel-mediated mechanosensation in the rat kidney. Am. J. Physiol. Physiol. 2015, 309, F369–F376.
- Jiang, Q.; Fu, X.; Tian, L.; Chen, Y.; Yang, K.; Chen, X.; Zhang, J.; Lu, W.; Wang, J. NOX4 Mediates BMP4-Induced Upregulation of TRPC1 and 6 Protein Expressions in Distal Pulmonary Arterial Smooth Muscle Cells. PLoS ONE 2014, 9, e107135.
- Kim, E.Y.; Anderson, M.; Dryer, S.E. Insulin increases surface expression of TRPC6 channels in podocytes: Role of NADPH oxidases and reactive oxygen species. Am. J. Physiol. Physiol. 2012, 302, F298–F307.
- Clempus, R.E.; Sorescu, D.; Dikalova, A.E.; Pounkova, L.; Jo, P.; Sorescu, G.P.; Schmidt, H.H.H.; Lassègue, B.; Griendling, K.K. Nox4 Is Required for Maintenance of the Differentiated Vascular Smooth Muscle Cell Phenotype. Arter. Thromb. Vasc. Biol. 2007, 27, 42–48.
- Hilenski, L.L.; Clempus, R.E.; Quinn, M.; Lambeth, J.D.; Griendling, K.K. Distinct Subcellular Localizations of Nox1 and Nox4 in Vascular Smooth Muscle Cells. Arter. Thromb. Vasc. Biol. 2004, 24, 677–683.
- Lee, M.; Martín, A.S.; Valdivia, A.; Martin-Garrido, A.; Griendling, K.K. Redox-Sensitive Regulation of Myocardin-Related Transcription Factor (MRTF-A) Phosphorylation via Palladin in Vascular Smooth Muscle Cell Differentiation Marker Gene Expression. PLoS ONE 2016, 11, e0153199.
- Martin-Garrido, A.; Brown, D.I.; Lyle, A.N.; Dikalova, A.; Seidel-Rogol, B.; Lassègue, B.; Martín, A.S.; Griendling, K.K. NADPH oxidase 4 mediates TGF-β-induced smooth muscle α-actin via p38MAPK and serum response factor. Free Radic. Biol. Med. 2011, 50, 354–362.
- Panico, C.; Luo, Z.; Damiano, S.; Artigiano, F.; Gill, P.; Welch, W.J. Renal Proximal Tubular Reabsorption Is Reduced In Adult Spontaneously Hypertensive Rats. Hypertension 2009, 54, 1291–1297.
- Liu, J.; Yan, Y.; Liu, L.; Xie, Z.; Malhotra, D.; Joe, B.; Shapiro, J.I. Impairment of Na/K-ATPase Signaling in Renal Proximal Tubule Contributes to Dahl Salt-sensitive Hypertension. J. Biol. Chem. 2011, 286, 22806–22813.
