Breast cancer (BC) is the most frequent cancer diagnosed in women worldwide. This heterogeneous disease can be classified into four molecular subtypes (luminal A, luminal B, HER2 and triple-negative breast cancer (TNBC)) according to the expression of the estrogen receptor (ER) and the progesterone receptor (PR), and the overexpression of the human epidermal growth factor receptor 2 (HER2). Current BC treatments target these receptors (endocrine and anti-HER2 therapies) as a personalized treatment. Along with chemotherapy and radiotherapy, these therapies can have severe adverse effects and patients can develop resistance to these agents. Moreover, TNBC do not have standardized treatments. Hence it is essential to develop new treatments to target more effectively each BC subgroup.
- breast cancer
- personalized therapies
- molecular subtypes
- breast cancer treatment
- luminal
- HER2
- TNBC
1. Introduction
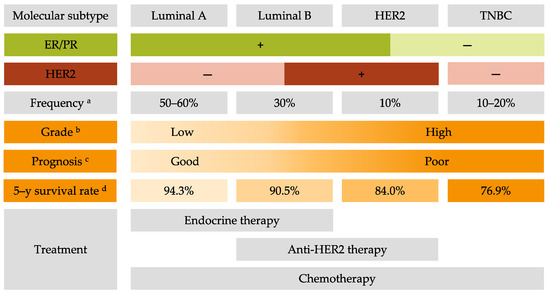
2. Common Treatments for All Breast Cancer Subtypes
In addition to surgery, radiotherapy and chemotherapy are used routinely to treat all BC subtypes [17].2.1. Surgery
2.2. Radiotherapy
2.3. Chemotherapy
3. Current Personalized Treatments for Breast Cancer: Strengths and Weaknesses
The current strategies of treatment are principally based on the tumor progression and BC molecular subtypes in order to offer the most personalized treatment for BC patients. The algorithm of BC treatment is represented in Figure 2.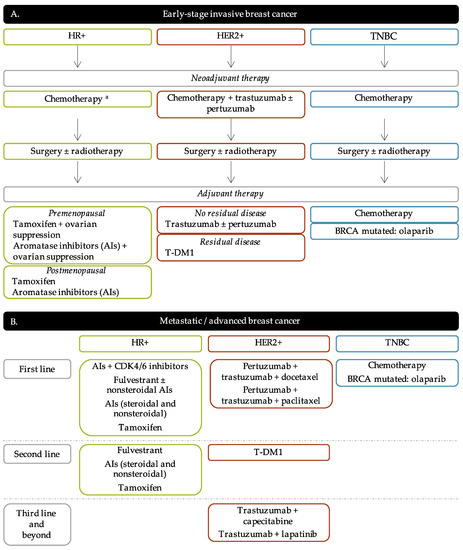
3.1. Endocrine Therapy
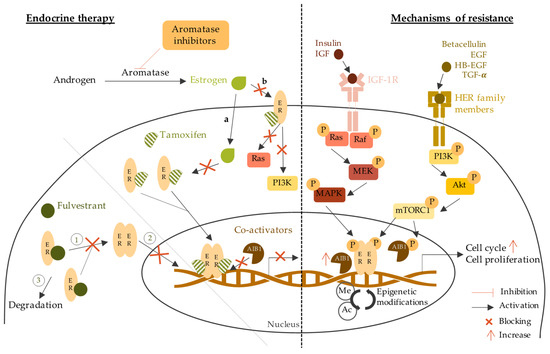
3.2. Anti-HER2 Therapy
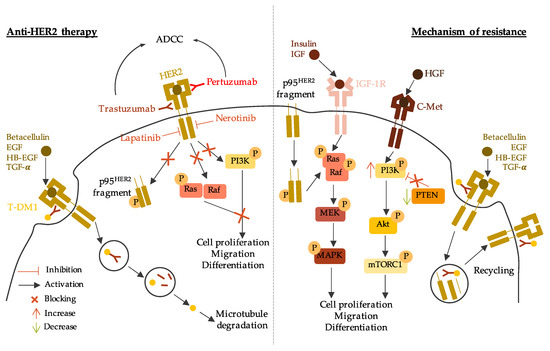
3.3. PARP Inhibitors
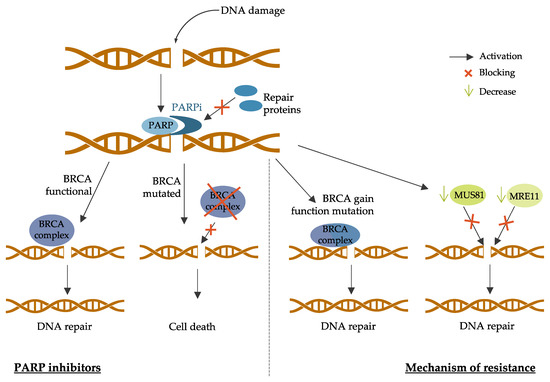
4. New Strategies and Challenges for Breast Cancer Treatment
4.1. Emerging Therapies for HR-Positive Breast Cancer
Targeted Therapy | Drug Name |
|---|
Targeted Therapy | Drug Name | Trial Number | Trial Number | Patient Population | Trial Arms | Outcomes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Drug Name | Patient Population | Trial Number | Patient Population | Trial Arms | Outcomes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Trial Arms | Outcomes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pan-PI3K inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Antibodies drug conjugate (ADC) | Buparlisib | Trastuzumab-deruxtcan | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Antibodies Drug Conjugate | (DS-8201a) | Sacituzumab govitecan | BELLE-2 | Phase III | NCT01610284 [ 41] | DESTINY-Breast01 | [ | Phase II | NCT03248492 [ 64] |
[ ] |
ASCENT | Phase III | ] |
NCT02574455 [ 81] |
[ ] | HR+/HER2- | Postmenopausal | Locally advanced or MBC | HER2+ Prior AI treatment | MBC | Buparlisib + fulvestrant vs. placebo + fulvestrant | Prior trastuzumab-emtansine treatment | PFS 6.9 months vs. 5.0 months (HR 0.78; p |
TNBC | MBC | Prior standard treatmentTrastuzumab-deruxtcan monotherapy | = 0.00021) |
Sacituzumab govitecan vs. single-agent chemotherapy | PFS 6.8 months vs. 4.0 months in PI3K mutated (HR 0.76; p = 0.014) | ||||||||||||||||||||||||||||||||
PFS 16.4 months | BELLE-3 | Phase III | NCT01633060 [ 42] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PFS 5.6 months vs. 1.7 months (HR 0.41; | p | < 0.001) | PFS 12.1 months vs. 6.7 months (HR 0.48; p < 0.001) |
[ ] |
Trastuzumab-duocarmycin (SYD985) | HR+/HER2- | Postmenopausal | Locally advanced or MBC | |||||||||||||||||||||||||||||||||||||||||||||||||||||
VEGF inhibitors | Phase I dose-escalation and dose-expansion | NCT02277717 Prior endocrine therapy or mTOR inhibitors |
[ 65] |
[ 265] | Buparlisib + fulvestrant vs. placebo + fulvestrant |
Bevacizumab | PFS 3.9 months vs. 1.8 months (HR 0.67; p = 0.0003) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
HER2+ | Locally advanced or metastatic solid tumors | BEATRICE | Phase III | NCT00528567 [ 82] |
[ ] | Trastuzumab-duocarmycin monotherapy | Early TNBC | Surgery | ORR 33% | BELLE-4 | Phase II/III | NCT01572727 [ 43] |
[ | ||||||||||||||||||||||||||||||||||||||||||||||||
Bevacizumab + chemotherapy vs. chemotherapy alone | IDFS 80% vs. 77% | OS 88% vs. 88% | ] |
Modified antibodies | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CALGB 40603 | Phase II | NCT00861705 [ | HER2- | Locally advanced or MBC | No prior chemotherapy | ] |
[ ] | Margetuxumab (MGAH22) | TNBC | Stage II to IIIBuparlisib + pacliatxel vs. placebo + paclitaxel | SOPHIA | Phase III | NCT02492711 [ 66] |
[ ] | PFS 8.0 months vs. 9.2 months (HR 1.18, 95% CI 0.82–1.68) | PFS 9.1 months vs. 9.2 months in PI3K mutated (HR 1.17, 95% 0.63–2.17) | |||||||||||||||||||||||||||||||||||||||||||||
HER2+ | Bevacizumab + chemotherapy vs. chemotherapy alone or Carboplatin + chemotherapy vs. chemotherapy alone Advanced or MBC | Prior anti-HER2 therapies | pCR 59% vs. 48% (p = 0.0089) (Bevacizumab) | pCR 60% vs. 44% ( p = 0.0018) (Carboplatin) | Margetuximab + chemotherapy vs. trastuzumab + chemotherapy | PFS 5.8 months vs. 4.9 months (HR 0.76; p = 0.03) | OS 21.6 months vs. 19.8 months (HR 0.89; p = 0.33) | ORR 25% vs. 14% ( p < 0.001) | Pictilisib | FERGI | Phase II | NCT01437566 [ 44] |
[ ] |
HR+/HER2- | Postmenopausal | Prior AI treatment | Pictilisib + fulvestrant vs. placebo + fulvestrant | PFS 6.6 months vs. 5.1 months (HR 0.74; p = 0.096) | PFS 6.5 months vs. 5.1 months in PI3K mutated (HR 0.74; p = 0.268) | PFS 5.8 months vs. 3.6 months in non-PI3K mutated (HR 0.72; p = 0.23) | |||||||||||||||||||||||||||||||||||||||||
PEGGY | Phase II | NCT01740336 [ 45] |
[ ] |
HR+/HER2- | Locally recurrent | or MBC | Pictilisib + paclitaxel vs. placebo + paclitaxel | PFS 8.2 months vs. 7.8 months (HR 0.95; p = 0.83) | PFS 7.3 months vs. 5.8 months in PI3K mutated (HR 1.06; p = 0.88) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Isoform-specific inhibitors | Alpelisib | Phase Ib | NCT01791478 [46] |
[ 235] |
HR+/HER2- | Postmenopausal | MBC | Prior endocrine therapy | Alpelisib + letrozole | CBR 35% (44% in patients with PIK3CA mutated and 20% in PIK3CA wild-type tumors; 95% CI [17%; 56%]) | |||||||||||||||||||||||||||||||||||||||||||||||||||
SOLAR-1 | Phase III | NCT02437318 [ 47] |
[ ] |
HR+/HER2- | Advanced BC | Prior endocrine therapy | Alpelisib + fulvestrant vs. placebo + fulvestrant | PFS 7.4 months vs. 5.6 months in non-PI3K mutated (HR 0.85, 95% CI 0.58–1.25) | PFS 11.0 months vs. 5.7 months in PI3K mutated (HR 0.65; p = 0.00065) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
NEO-ORB | Phase II | NCT01923168 [ 48] |
[ ] |
HR+/HER2- | Postmenopausal | Early-stage BC | Neoadjuvant setting | Alpelisib + letrozole vs. placebo + letrozole | ORR 43% vs. 45% (PIK3CA mutant), 63% vs. 61% (PIK3CA wildtype) | pCR rates low in all groups | |||||||||||||||||||||||||||||||||||||||||||||||||||
Taselisib | SANDPIPER | Phase III | NCT02340221 [ 49] |
[ ] |
HR+/HER2- | Postmenopausal | Locally advanced or MBC | PIK3CA-mutant | Prior AI treatment | Taselisib + fulvestrant vs. placebo + fulvestrant | PFS 7.4 months vs. 5.4 months (HR 0.70; p = 0.0037) | ||||||||||||||||||||||||||||||||||||||||||||||||||
LORELEI | Phase II | NCT02273973 [ 50] |
[ ] |
HR+/HER2- | Postmenopausal | Early-stage BC | Neoadjuvant setting | Taselisib + letrozole vs. placebo + letrozole | ORR 50% vs. 39.3% (OR 1.55; p = 0.049) | ORR 56.2% vs. 38% in PI3K mutated (OR 2.03; p = 0.033) | No significant difference in pCR | ||||||||||||||||||||||||||||||||||||||||||||||||||
mTOR inhibitors | Everolimus | BOLERO-2 | Phase III | NCT00863655 [ 51] |
[ ] |
HR+/HER2- | Advanced BC | Prior AI treatment | Everolimus + exemestane | vs. placebo + exemestane | PFS 6.9 months vs. 2.8 months (HR 0.43; p < 0.001) | ||||||||||||||||||||||||||||||||||||||||||||||||||
TAMRAD | Phase II | NCT01298713 [ 52] |
[ ] |
HR+/HER2- | Postmenopausal | MBC | Prior AI treatment | Everolimus + tamoxifen vs. tamoxifen alone | CBR 61% vs. 42% | TTP 8.6 months vs. 4.5 months (HR 0.54) | |||||||||||||||||||||||||||||||||||||||||||||||||||
Tyrosine kinase inhibitors | Tucatinib | HER2CLIMB | Phase II | NCT02614794 [ 67] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EGFR inhibitors | Cetuximab |
[ ] |
TBCRC 001 | Phase II | NCT00232505 [ 84] |
[ ] | HER2+ | Locally advanced or MBC | Prior anti-HER2 therapies | TNBC | MBC | Tucatinib + trastuzumab and capecitabine vs. placebo + trastuzumab and capecitabine | Cetuximab + carboplatin | PFS 33.1% (7.8 months) vs. 12.3% (5.6 months) (HR 0.54; p < 0.001) | PFS 24.9% vs. 0% (HR 0.48; p < 0.001) in brain metastases patients | OS 44.9% vs. 26.6% (HR 0.66; p = 0.005) | |||||||||||||||||||||||||||||||||||||||||||||
Response < 20% | TTP 2.1 months | Poziotinib | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Phase II | NCT00463788 [85] |
[ 313] | NOV120101-203 | Phase II | NCT02418689 [ |
TNBC ] |
[ ] |
MBC | Prior chemotherapy treatment | HER2+ | MBC | Prior chemotherapy and trastuzumab | Cetuximab + cisplatin vs. cisplatin alone | Poziotinib monotherapy | PFS 4.04 months | ||||||||||||||||||||||||||||||||||||||||||||||
ORR 20% vs. 10% ( | p | = 0.11) | PFS 3.7 months vs. 1.7 months (HR 0.67; p = 0.032) | OS 12.9 months vs. 9.4 months (HR 0.82; p = 0.31) | HER2-derived peptide vaccine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
mTORC1 inhibitors | E75 (NeuVax) | Everolimus | Phase I/II | NCT00841399 | NCT00854789 [ 69] |
[ ] |
Phase II | NCT00930930 [86] |
[ 314] | HER2+ | Node-positive or high-risk node-negative BC | HLA2/3+ | E75 vaccination vs. non-vaccination | TNBC | Stage II or III | Neoadjuvant treatment | Everolimus + cisplatin and paclitaxel vs. placebo + cisplatin and paclitaxel | DFS 89.7% vs. 80.2% (p = 0.008) | DFS 94.6% in optimal dosed patients ( p = 0.005 vs. non-vaccination) | ||||||||||||||||||||||||||||||||||||||||||
pCR 36% vs. 49% | GP2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Akt inhibitors | Phase II | NCT00524277 [70] |
[ 270] |
HER2 (IHC 1-3+) | Disease free | Node-positive or high-risk node-negative BC | HLA2+ | GP2 + GM-CSF vs. GM-CSF alone | DFS 94% vs. 85% (p = 0.17) | DFS 100% vs. 89% in HER2-IHC3+ ( p = 0.08) | |||||||||||||||||||||||||||||||||||||||||||||||||||
Ipatasertib | LOTUS | Phase II | NCT02162719 [ 87] |
[ ] |
TNBC | Locally advanced or MBC | No prior sytemic therapy | Ipatasertib + paclitaxel vs. placebo + paclitaxel | PFS 6.2 months vs. 4.9 months (HR 0.60; |
AE37 | Phase II | NCT00524277 [71] |
[ 271] |
HER2 (IHC 1-3+) | Node-positive or high-risk node-negative BC | AE37 + GM-CSF vs. GM-CSF alone | DFS 80.8% vs. 79.5% (p = 0.70) | DFS 77.2% vs. 65.7% ( p = 0.21) HER2-low | DFS 77.7% vs. 49.0% ( p = 0.12) TNBC | ||||||||||||||||||||||||||||||||||||||||||
p | = 0.037) | PFS 6.2 months vs. 3.7 moths (HR 0.58; | p | = 0.18) in PTEN-low patients | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
FAIRLANE | Phase II | NCT02301988 [ 88] |
[ ] |
Early TNBC | Neoadjuvant treatment | Ipatasertib + paclitaxel vs. placebo + paclitaxel | pCR 17% vs. 13% | pCR 16% vs. 13% PTEN-low patients | pCR 18% vs. 12% PIK3CA/AKT1/PTEN-altered patients | PI3K inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||
Capivasertib | Alpelisib | PAKT | Phase II | NCT02423603 [ 89] |
[ ] | Phase I | NCT02167854 [72] |
[ 272] |
TNBC | MBC | No prior chemotherapy treatment | HER2+ | Capivasertib + paclitaxel vs. placebo + paclitaxel MBC with a PIK3CA mutation Prior ado-trastuzumab emtansine and pertuzumab | Alpelisib + Trastuzumab + LJM716 | PFS 5.9 months vs. 12.6 months (HR 0.61; Toxicities limited drug delivery 72% for alpelisib 83% for LJM716 | ||||||||||||||||||||||||||||||||||||||||||||||
p | = 0.04) | Phase I | NCT02038010 [73] |
[ 273] |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Androgen receptor inhibitors | HER2+ | MBC | Prior trastuzumab-based therapy | Bicalutamide | Alpelisib + T-DM1 | Phase II | NCT00468715 [90] |
[ | PFS 8.1 months | ORR 43% | CBR 71% and 60% in prior T-DM1 patients | ||||||||||||||||||||||||||||||||||||||||||||||||||
] | HR- | AR+ or AR- | MBC | Bicalutamide monotherapy | CBR 19% | PFS 12 weeks | Copanlisib | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzalutamide | PantHER | Phase Ib | NCT02705859 [ 74] | Phase II | NCT01889238 [91] | [ ] |
[ 319] | HER2+ | Advanced BC | Prior anti-HER2 therapies | TNBC | AR+ | Locally advanced or MBC | Copanlisib + trastuzumab | Stable disease 50% | ||||||||||||||||||||||||||||||||||||||||||||||
Enzalutamide monotherapy | CBR 25% | OS 12.7 months | mTOR inhibitors | Everolimus | BOLERO-1 | Phase III | NCT00876395 [ 75] |
[ ] |
HER2+ | Locally advanced BC | No prior treatment | Everolimus + trastuzumab vs. placebo + trastuzumab | |||||||||||||||||||||||||||||||||||||||||||||||||
CYP17 inhibitors | PFS 14.95 months vs. 14.49 months (HR 0.89; | p = 0.1166) | PFS 20.27 months vs. 13.03 months (HR 0.66; p = 0.0049) | PrE0102 | Phase II | NCT01797120 [ 53] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Abiraterone acetate | UCBG 12-1 | Phase II | NCT01842321 [ 92] |
[ ] |
TNBC | AR+ | Locally advanced or MBC | Centrally reviewed | Prior chemotherapy | Abiraterone acetate + prednisone | CBR 20% | ORR 6.7% | PFS 2.8 months | BOLERO-3 | Phase III | [ ] |
NCT01007942 [ 76] |
[ ] | HR+/HER2- | Postmenopausal | MBC | Prior AI treatment | HER2+ | Advanced BC | Trastuzumab-resistant | Everolimus + fulvestrant | vs. placebo + fulvestrant | Prior taxane therapy | PFS 10.3 months vs. 5.1 months (HR 0.61; |
||||||||||||||||||||||||||||||||
Anti-PDL1 antibodies | Atezolizumab | Everolimus + trastuzumab and vinorelbine vs. placebo + trastuzumab and vinorelbine | Impassion 130 | Phase III | NCT02425891 [ 93] |
[ ] | p = 0.02) | CBR 63.6% vs. 41.5% ( p = 0.01) | |||||||||||||||||||||||||||||||||||||||||||||||||||||
PFS 7.00 months vs. 5.78 months (HR 0.78; | p | = 0.0067) | TNBC | Locally advanced or MBC | No prior treatment | Atezolizumab + nab-paclitaxel vs. placebo + nab-paclitaxel | OS 21.0 months vs. 18.7 months (HR 0.86; p = 0.078) | OS 25.0 months vs. 18.0 months (HR 0.71, 95% CI 0.54–0.94)) in PDL-1+ patients | Akt inhibitors | Capivasertib | |||||||||||||||||||||||||||||||||||||||||||||||||||
CDK4/6 inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Impassion 031 | Phase III | NCT03197935 [ 94] |
[ ] | FAKTION | Phase II | NCT01992952 |
TNBC | Stage II to III | No prior treatment |
[ ] |
[ ] |
Palbociclib | SOLTI-1303 PATRICIA | Phase II | NCT02448420 [ 77] |
[ ] |
Atezolizumab + chemotherapy vs. placebo + chemotherapy | HR+/HER2- | pCR 95% vs. 69% Postmenopausal | Locally advanced or MBC | Prior AI treatment | HER2+ | ER+ or ER- | p MBC | = 0.0044 Prior standard therapy including trastuzumab | Capivasertib + fulvestrant vs. placebo + fulvestrant | PFS 10.3 months vs. 4.8 months (HR 0.57; p = 0.0035) | ||||||||||||||||||||||||||||||||||
Palbociclib + trastuzumab | PFS 10.6 months (luminal) vs. 4.2 months (non-luminal) (HR 0.40; | p = 0.003) | Phase I | NCT01226316 [55] |
[ 244] |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ribociclib | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Durvalumab | ER+ | AKT1 E17K-mutant | MBC | Prior endocrine treatment | Phase Ib/II | NCT02657343 [78] |
[ 278] | Capivasertib + fulvestrant vs. Capivasertib alone | GeparNuevo | Phase II | NCT02685059HER2+ | Advanced BC | Prior treatment with trastuzumab, pertuzumab, and trastuzumab emtansine | CBR 50% vs. 47% | ORR 6% (fulvestrant-pretreated) and 20% (fulvestrant-naïve) vs. 20% | ||||||||||||||||||||||||||||||||||||||||||||||
Ribociclib + trastuzumab |
[ ] |
[ ] | PFS 1.33 months | TNBC | MBC | Stromal tumor-infiltrating lymphocyte (sTILs) | Durvalumab vs. placebo | No dose-limiting toxicities | pCR 53.4% vs. 44.2% | pCR 61.0% vs. 41.4% in window cohort | CDK4/6 inhibitors | ||||||||||||||||||||||||||||||||||||||||||||||||||
SAFIRO BREAST-IMMUNO | Phase II | NCT02299999 [ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Abemaciclib | Palcociclib | ] |
[ | MonarcHER | Phase II | NCT02675231 [ 79] |
[ ] | PALOMA-1 | Phase II | NCT00721409 [ 56] |
[ ] |
HR+/HER2- | Postmenopausal | Advanced BC | No prior systemic treatment | Palbocilib + letrozole vs. letrozole alone | HER2+ | Locally advanced or MBC | Prior anti-HER2 therapies | Abemaciclib + trastuzumab and fulvestrant (A) vs. abemaciclib + trastuzumab (B) vs. standard-of-care chemotherapy + trastuzumab (C) | PFS 8.3 months (A) vs. 5.7 months (C) (HR 0.67; p = 0.051) | PFS 5.7 months (B) vs. 5.7 months (C) (HR 0.97; p = 0.77) | PFS 20.2 months vs. 10.2 months (HR 0.488; p = 0.0004) | PFS 26.1 months vs. 5.7 months (HR 0.299; p < 0.0001) in non-Cyclin D1 amplified | PFS 18.1 months vs. 11.1 months (HR 0.508; p = 0.0046) in Cyclin D1 amplified | ||||||||||||||||||||||||||||||||||||
PALOMA-2 | Phase III | NCT01740427 [ 57] |
[ ] |
HR+/HER2- | Postmenopausal | Advanced BC | No prior systemic treatment | Palbocilib + letrozole vs. placebo + letrozole | PFS 24.8 months vs. 14.5 months (HR 0.58; p < 0.001) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
PALOMA-3 | Phase III | NCT01942135 [ 58] |
[ ] |
HR+/HER2- | MBC | Prior endocrine therapy | Palbociclib + fulvestrant | vs. placebo + fulvestrant | PFS 9.5 months vs. 4.6 months (HR 0.46; p < 0.0001) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Ribociclib | MONALEESA-2 | Phase III | NCT01958021 [ 59] |
[ ] |
HR+/HER2- | Postmenopausal | Advanced or MBC | Ribociclib + letrozole vs. placebo + letrozole | PFS 25.3 months vs. 16.0 months (HR 0.568; p < 0.0001) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
MONALEESA-3 | Phase III | NCT02422615 [ 60] |
[ ] |
HR+/HER2- | Advanced BC | No prior treatment or prior endocrine therapy | Ribociclib + fulvestrant vs. placebo + fulvestrant | PFS 20.5 months vs. 12.8 months (HR 0.593; p < 0.001) | |||||||||||||||||||||||||||||||||||||||||||||||||||||
Abemaciclib | MONARCH-2 | Phase III | NCT02107703 [ 61] |
[ ] |
HR+/HER2- | Advanced or MBC | Prior endocrine treatment | Abemaciclib + fulvestrant vs. fulvestrant alone | PFS 16.4 months vs. 9.3 months (HR 0.553; p < 0.001) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
MONARCH-3 | Phase III | NCT02246621 [ 62] |
[ ] |
HR+/HER2- | Advanced or MBC | Prior endocrine treatment | Abemaciclib + anastrozole or letrozole vs. placebo + anastrozole or letrozole | PFS 28.18 months vs. 14.76 months (HR 0.546; p < 0.0001) |
HR+: hormone receptors positive; HER2-: human epidermal growth factor receptor 2 negative; MBC: metastatic breast cancer; BC: breast cancer; PFS: progression free survival; CBR: clinical benefit rate; ORR: objective response rate; pCR: pathologic complete response; HR: hazard ratio.
4.2. New Strategic Therapies for HER2-Positive Breast Cancer
HER2+: human epidermal growth factor receptor 2 positive; ER+: estrogen receptor positive; HLA2/3: human leucocyte antigen 2/3; MBC: metastatic breast cancer; BC: breast cancer; PFS: progression free survival; CBR: clinical benefit rate; ORR: objective response rate; DFS: disease-free survival OS: overall survival GM-CSF: granulocyte macrophage colony-stimulated factor; HR: hazard ratio.
4.3. Emerging Therapies for Triple Negative Breast Cancer (TNBC)
Targeted Therapy | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
] | |||||||||||||||||||||||||||||||||||||||||
HER2- | |||||||||||||||||||||||||||||||||||||||||
MBC | |||||||||||||||||||||||||||||||||||||||||
Prior chemotherapy | |||||||||||||||||||||||||||||||||||||||||
Durvalumab vs. maintenance chemotherapy | |||||||||||||||||||||||||||||||||||||||||
HR of death 0.37 for PDL-1+ patients | HR of death 0.49 for PDL-1- patients | ||||||||||||||||||||||||||||||||||||||||
Phase I | NCT02484404 [97] |
[ 325] |
Recurrent women’s cancers including TNBC | Durvalumab + cediranib + olaparib | Partial response 44% | CBR 67% | |||||||||||||||||||||||||||||||||||
Avelumab | JAVELIN | Phase Ib | NCT01772004 [ 98] |
[ ] |
MBC | Prior standard-of-care therapy | Avelumab monotherapy | ORR 3.0% overall | ORR 5.2% in TNBC | ORR 16.7% in PDL-1+ vs. 1.6% in PDL-1- overall | ORR 22.2.% in PDL-1+ vs. 2.6% in PDL-1- in TNBC | ||||||||||||||||||||||||||||||
Anti-PD1 antibodies | Pembrolizumab | KEYNOTE-086 | Phase II | NCT02447003 [ 99] |
[ ] |
TNBC | MBC | Prior or no prior systemic therapy | Pembrolizumab monotherapy | Previously treated patients: | ORR 5.3% overall | ORR 5.7% PDL-1+ patients | PFS 2.0 months | OS 9.0 months | Non-previously pretreated: | ORR 21.4% | PFS 2.1 months | OS 18.0 months | |||||||||||||||||||||||
KEYNOTE-119 | Phase III | NCT02555657 [ 100] |
[ ] |
TNBC | MBC | Prior systemic therapy | Pembrolizumab vs. chemotherapy | OS 12.7 months vs. 11.6 months (HR 0.78; p = 0.057) in PDL1+ patients | OS 9.9 months vs. 10.8 months (HR 0.97, 95% CI 0.81–1.15) | ||||||||||||||||||||||||||||||||
KEYNOTE-355 | Phase III | NCT02819518 [ 101] |
[ ] |
TNBC | MBC | No prior systemic therapy | Pembrolizumab + chemotherapy vs. placebo + chemotherapy | PFS 9.7 months vs. 5.6 months (HR 0.65; p = 0.0012) in PDL-1+ patients | PFS 7.6 months vs. 5.6 months (HR 0.74; p = 0.0014) | ||||||||||||||||||||||||||||||||
KEYNOTE-522 | Phase III | NCT03036488 [ 102] |
[ ] |
Early TNBC | Stage II to III | No prior treatment | Pembrolizumab + paclitaxel and carboplatin vs. placebo + paclitaxel and carboplatin | pCR 64.8% vs. 51.2 % (p < 0.001) | |||||||||||||||||||||||||||||||||
Anti-CDL4 antibodies | Tremelimumab | Phase I [103] |
[331] |
Incurable MBC | Tremelimumab + radiotherapy | OS 50.8 months | |||||||||||||||||||||||||||||||||||
Vaccines | PPV | Phase II | UMIN000001844 [104] |
[ 332] |
TNBC | MBC | Prior systemic therapy | PPV vaccine | PFS 7.5 months | OS 11.1 months | |||||||||||||||||||||||||||||||
STn-KLH | Phase III | NCT00003638 [105] |
[ 333] |
MBC | Prior chemotherapy | Partial or complete response | STn-KLH vaccine vs. non-vaccine | TTP 3.4 months vs. 3.0 months |
TNBC: triple negative breast cancer; HER2: human epidermal growth factor receptor; HR: hormonal receptor; MBC: metastatic breast cancer; BC: breast cancer; AR: androgen receptor; PPV: personalized peptide vaccine; PFS: progression free survival; CBR: clinical benefit rate; ORR: objective response rate; IDFS: invasive disease-free survival; OS: overall survival; TTP: time to progression; pCR: pathologic complete response; HR: hazard ratio.
