Advanced oxidation processes (AOPs) are water treatment processes that are promising for the degradation of persistent or toxic organic pollutants, as well as compounds refractory to other environmental remediation/decontamination treatments. AOPs have gained great importance as alternative treatment processes that affect the degradation of organic species through the action of the hydroxyl radical (OH), oxidizing pollutants present in wastewater and industrial effluents. AOPs are carried out at room temperature and at a pressure close to normal, which involve the formation of very reactive radical species with a high oxidizing capacity, mainly hydroxyl (OH) radicals. These OH radicals are extremely reactive oxidizers (oxidation potential of the OH radical is approximately, Eθ = 2.8 V) and non-selective towards organic pollutants in wastewater. AOPs can be considered versatile technologies, as they provide different possible alternatives to produce OH radicals. AOPs, compared to conventional water treatment techniques, have a greater efficiency and capacity to degrade recalcitrant organic pollutants, and can generate less toxic intermediate products during their degradation.
- AOPs
- water treatment
1. UV/H2O2 Processes
2
2
2
2), leading to its photolysis, in order to generate two hydroxyl (OH) radicals [1], as illustrated in following equation [2]:
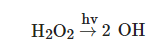 H2O2→hv2 OH
H2O2→hv2 OHIn addition to direct photolysis, the UV/H
2
2
2
2
2
2
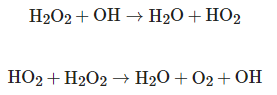 HO2+H2O2→H2O+O2+OH
HO2+H2O2→H2O+O2+OHThe efficiency of OH radical production depends on the ability of hydrogen peroxide to absorb UV radiation, as well as the physical and chemical characteristics of the fluid that will be subjected to the oxidation process. UV absorption by H
2
2
2
2, increasing the production potential of the OH radical [2].
2
2
2
2 system is currently one of the most promising technologies for wastewater treatment [2][4].
2. Persulfate Based Processes
2
82−) is a new AOP, which has been shown to be a very promising alternative for water and wastewater treatment [5][6].
θ = 2.01 V), persulfate is considered an emerging oxidant in the degradation of pollutants present in water, moreover, persulfate is relatively stable at room temperature and has a non-selective behavior in regarding the degradation of pollutants [7][8]. Persulfate can also be activated to produce the sulfate radical (SO
4
θ
2
82− [9].
4 radical can be generated through heat, ultraviolet light, alkali, ultrasound, transition metal ions and activation of metal oxides [6][10].
4 radical has a longer shelf life, has a wider pH range of action, proved to be more stable and effective in oxidation for water decontamination [11][12]. Furthermore, some studies suggest that after the generation of the SO
4 radical, it may react with several species in solution to form other active species, such as the OH radical, which will play a key role in the pollutant degradation process [9][12].
3. Chlorine and NH2Cl Based Processes
Chlorine is one of the most used chemical oxidants worldwide for the disinfection of drinking water [13]. Currently, the UV/chlorine process is considered an emerging AOP, constituting an alternative to the UV/H
2
2 process in water treatment, effective in the degradation of a variety of persistent contaminants, such as desethylatrazine, sulfamethoxazole, carbamazepine, diclofenac, benzotriazole, tolyltriazole, iopamidol, 17α-ethinylestradiol [14][15]. Compared to the UV/H
2
2 process, the UV/chlorine process demonstrated greater efficiency in the degradation of some micropollutants under slightly acidic conditions, such as trichloroethylene [16]. In the UV/chlorine process, reactive species such as the OH radical and the Cl radical are formed from photolysis of free chlorine (HOCl/OCl-), as shown in the following equations [15]:
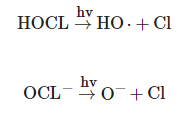 HOCL→hvHO⋅+Cl
HOCL→hvHO⋅+Cl2•−
2•− are strong oxidizers with redox potentials of 2.4 and 2.0 V, respectively. The various reactive species, including the OH radical, formed during this process make the UV/chlorine process a promising AOP for controlling a variety of contaminants in water treatment [14]. Furthermore, chlorine that does not react after the UV/chlorine process can provide residual protection in water distribution systems [17].
2Cl) has also attracted significant interest as a new AOP for the degradation of emerging water contaminants, such as carbamazepine, and for efficiently controlling the formation of disinfection by-products [13]. Furthermore, NH
2Cl is considered adequate to provide residual disinfection throughout the water distribution system due to its high stability [18].
2
2
2 radical, there is still not much information about its reactivity to pollutants present in water [19]. Primary radicals can further react with water co-solutes to form secondary radicals (such as CO
3•-
2•-
2Cl process demonstrates considerable potential as a new AOP for water treatment [13][19].
4. Fenton Processes
The Fenton process consists of the formation of OH radicals in an acidic medium, from the decomposition of hydrogen peroxide by the action of an iron catalyst [20]. The Fenton process is a very promising AOP due to the high mineralization promoted under normal conditions of both temperature and pressure, very effective in destroying refractory and toxic organic pollutants present in wastewater [21][22]. The main reactions involved in Fenton processes are [23]:
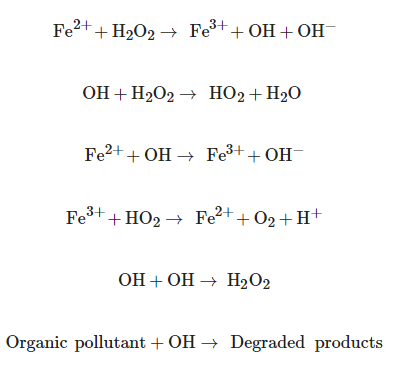 Fe2++H2O2→ Fe3++OH+OH−
Fe2++H2O2→ Fe3++OH+OH−The advantage of Fenton processes is due to the fact that they do not require sophisticated equipment or expensive reagents, they are considered ecologically viable processes due to their high performance and their relatively simple approach, which uses less harmful chemicals and cyclical nature, being necessary a lower concentration of these chemicals [23].
A condition of this AOP is the restricted pH range, where the generation of OH radicals during the Fenton reaction is only efficient under acidic pH conditions (with a pH value close to 3) [21][22].
There are different types of Fenton processes, including Fenton, photo-Fenton, electro-Fenton, photo-electro-Fenton, homogeneous and heterogeneous Fenton, among others. Among the various AOPs, it was the Fenton and photo-Fenton processes that proved to be the most effective, energy efficient and least expensive methods for treating recalcitrant compounds, when used exclusively or in conjunction with other conventional and biological methods [23].
5. Ozone Based Processes
3
3
θ = 2.07 V), leading to the destruction or degradation of organic pollutants, through different pathways, namely molecular ozone (direct) or through the hydroxyl radical (indirect) [24]. The main semi-reactions of ozone in water are [25]:
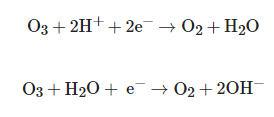 O3+2H++2e−→O2+H2O
O3+2H++2e−→O2+H2O3, in addition to being a relatively slow reaction, is also more selective than indirect reactions [26]. In water, O
3 undergoes a series of reactions, decomposing into several oxidative species, including the OH radical, which is a stronger oxidant than the original molecular ozone, which reacts with organic and inorganic compounds in a non-selective way, with rates very high reaction rates. Thus, the high oxidative power of ozonization is partially due to the generation of OH radicals [25].
3
2
2
3/UV), or addition of metallic catalysts, increasing the efficiency of the treatment. These processes are called advanced oxidation processes (AOPs). Thus, ozonation and ozone-based AOPs are responsible for the destruction of many recalcitrant organic compounds in water and wastewater, including pharmaceuticals and personal care products, solvents, surfactants and pesticides [24][25].
6. Heterogeneous Photocalalytic Processes
Heterogeneous photocatalysis is an AOP that has been widely studied in the last two decades. The principle of heterogeneous photocatalysis is associated with the activation of a semiconductor by the action of light, when the semiconductor and the reagent are in different phases, photocatalytic reactions are classified as heterogeneous photocatalysis [27].
2 and water [28].
2
2 has high chemical and thermal stability, is non-toxic, inexpensive and has a relatively high efficiency [27].
As a green, highly efficient and ecological technology, heterogeneous photocatalysis for the treatment of organic pollutants present in wastewater has been shown to be a promising technology to face future environmental challenges [29].
