Bioethanol has many environmental and practical benefits as a transportation fuel. It is one of the best alternatives to replace fossil fuels due to its liquid nature, which is similar to the gasoline and diesel fuels traditionally used in transportation. In addition, bioethanol production technology has the capacity for negative carbon emissions, which is vital for solving the current global warming dilemma. However, conventional bioethanol production takes place based on an inland site and relies on freshwater and edible crops (or land suitable for edible crop production) for production, which has led to the food vs. fuel debate. Establishing a coastal marine biorefinery (CMB) system for bioethanol production that is based on coastal sites and relies on marine resources (seawater, marine biomass and marine yeast) could be the ultimate solution. In this paper, we aim to evaluate the environmental impact of using seawater for bioethanol production at coastal locations as a step toward the evaluation of a CMB system. Hence, a life cycle assessment for bioethanol production was conducted using the proposed scenario, named Coastal Seawater, and compared to the conventional scenario, named Inland Freshwater (IF). The impact of each scenario in relation to climate change, water depletion, land use and fossil depletion was studied for comparison. The Coastal Seawater scenario demonstrated an improvement upon the conventional scenario in all the selected impact categories. In particular, the use of seawater in the process had a significant effect on water depletion, showing an impact reduction of 31.2%. Furthermore, reductions were demonstrated in natural land transformation, climate change and fossil depletion of 5.5%, 3.5% and 4.2%, respectively. This indicates the positive impact of using seawater and coastal locations for bioethanol production and encourages research to investigate the CMB system.
- bioethanol
- LCA
- marine fermentation
- seawater
- Saccharomyces cerevisiae
- water footprint
- bioenergy
- biofuel
- marine yeast
- GHG
- environment
- SimaPro
- net zero
1. Introduction
Growing concern regarding the effect of anthropogenic activity on climate change has given impetus to research greener energy sources. Governments worldwide, including the UK, are committed to reaching climate change goals detailed in the Paris Agreement [1]. To reach these goals it is essential to base infrastructure on a framework of sustainable technology, not only to reduce emissions but also to mitigate environmental changes already generated such as long-lived carbon dioxide (CO2) emissions.
Bioethanol is a liquid renewable biofuel that is widely used in blends with gasoline to improve the octane rating of the vehicle’s engine and reduce carbon footprint. It is produced by microbial fermentation of sugars, and these sugars are sourced from biological materials such as sugary and starchy crops (1st generation bioethanol), lignocellulosic biomass (2nd generation bioethanol) and seaweed (3rd generation bioethanol). During cultivation, these biological materials absorb CO2 from the atmosphere by photosynthetic growth. Furthermore, fermentation of the derived sugars produces a highly pure stream of CO2 and can thus be integrated with carbon capture and storage (CCS) technology [2]. Together with CCS, bioethanol production can achieve negative emissions necessary to help in reaching the UN’s climate goals. In addition, there are many pathways for carbon capture, storage and utilisation (CCSU), each coming with their own environmental footprints [3]. It has been demonstrated that when linked with carbon sequestration, bioethanol production can be carbon negative. However, it is important that the CCS and carbon utilisation pathways are selected with high regard to the environmental impact. For example, if the captured carbon were to be used in enhanced oil recovery, the carbon captured during the bioethanol production process would be brought to zero or even surpassed by the carbon release in combustion of the mined oil.
Although bioethanol is regarded as a promising alternative fuel, there are some economic, policy and environmental issues associated with the current production processes. For example, a lack of policy to support ethanol-petrol blending in many countries results in a lack of demand as well as high production costs. Whilst the US government mandates supply of E10 at a minimum, the petrol served in the UK is not regulated and is generally only E5. A production process incurring lesser cost or one that offsets costs with greater earning potential would encourage bioethanol production. This lack of policy support is mainly due to the food security vs. biofuel feedstock supply debate which regards bioethanol production as a threat to the food supply as it consumes the arable land and freshwater that are vital for producing food crops. A new pathway for bioethanol production that reduces freshwater consumption and arable land use would achieve social acceptance and encourage policymakers to legislate policies that support bioethanol production and utilisation.
Weak ethanol-petrol blends such as E10 and E15 (10 and 15% ethanol) can be safely and readily introduced into supply [4]. It has been shown that blending petrol with ethanol lowers emissions during combustion [5, 6[5][6]]. The UK government estimated that using the E10 ethanol blend in the UK could cut CO2 from road transport by 750,000 tonnes per year which is equivalent to removing 350,000 cars off roads [7]. Ethanol use in combustion engines offers reductions in emissions and improvements in power and energy efficiency compared to fossil fuels such as diesel and petrol [8]. Additionally, ethanol is biodegradable and evaporates in open spaces [9]. This means it is unlikely to cause significant damage, environmental or otherwise, upon spillage.
Transforming land for biofuel feedstock cultivation in addition to that necessary for food production, a phenomenon known as land use change (LUC), comes with a severe environmental impact [10] and prompts concerns over food security. For this reason, the Renewable Energy Directive (EU) imposed a 7% ceiling on the use of bioenergy for transportation where derived from food crops in 2018 [11]. This has prompted a transition from the first-generation biofuels derived from food-based feedstocks such as corn (used in the USA), sugarcane (used in Brazil), wheat and sugar beet (used in the UK), to the second-generation biofuels which are derived from non-food lignocellulosic biomass such as wheat straw, switchgrass, willow and miscanthus. Currently in-development are third-generation biofuels produced using algal and waste feedstocks requiring no land.
Biofuels can have greater water footprint (WF) than fossil fuels [12], highlighting the importance of considering a fuel product’s impact across its entire life cycle rather than only in combustion. The WF of first-generation bioethanol ranges from 1400 to 9800 litre (L) of freshwater per L of ethanol [13[13][14], 14], hence it presents an environmental concern. With much of the world facing water scarcity, there is understandable resistance to bioethanol at the cost of water supply. Although second-generation bioethanol from crop residues demonstrates much lower WF [15], the WF can be reduced further by focusing on the entire process rather than feedstock only. One way to do that is to utilise seawater instead of freshwater for preparing the fermentation medium for bioethanol production. This suggests the construction of biorefinery facilities in a coastal location and using marine yeast strains for fermentation.
Seawater is non-potable and abundant, therefore its use in industrial scale fermentation reduces the WF of the product and would not detract from general water supply [16]. It is also freely available and thus offers a reduction in production cost. Halotolerant yeasts, sourced in the marine environment, would be applied in conjunction with seawater use. This is because they demonstrate improved function in seawater-based fermentation medium as compared to terrestrial yeasts [13, 17][13][17]. The marine yeast strain S. cerevisiae AZ65 produced bioethanol at a higher productivity rate and improved yield using freshwater-YPD medium and a similar productivity rate and improved yield using seawater-YPD medium compared to the industrial terrestrial strain S. cerevisiae NCYC2592. S. cerevisiae AZ65 was also able to produce bioethanol efficiently using seawater-molasses medium [13, 18][13][18].
The aim of this study is to quantify the environmental and cost impacts of bioethanol production using seawater fermentation at a coastal site. The preliminary life cycle and technoeconomic analysis of bioethanol explores three elements: coastal location, seawater medium and marine yeasts. A comparative study assesses the proposed coastal-seawater system as opposed to a conventional bioethanol production system from sugar beet.
2. Analysis on Results
The life cycle impact assessment used the ReCiPe Midpoint Hierachist method in SimaPro with 18 impact categories by default. The Inland-Freshwater scenario was used as the base case scenario and values of all impact categories were normalised as 100% for comparison. The Coastal-Seawater scenario demonstrated reductions in 15 of these impact categories and was the same in the remaining three impact categories relative to the Inland-Freshwater scenario. The environmental impact reduction was primarily in the water depletion category with a decrease of 31.2%. Climate change, natural land transformation, urban land occupation and fossil depletion were all reduced by 3.5–5.5%. Agricultural land occupation showed no change as the modelled first-generation feedstock production relies on arable land in both scenarios (Figure 1).
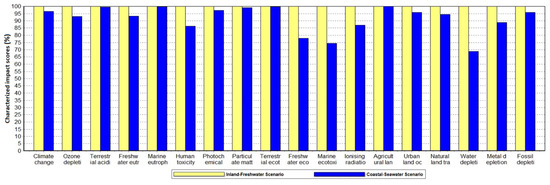
Figure 1. Complete life cycle environmental impacts of the two scenarios of bioethanol production. The data have been characterized for direct comparison of the scenarios in each impact category based on ethanol (95% in solution state) from sugar beet, Alloc Def, U.
The Climate Change impact category describes the effect of the system on global temperature reported in ‘year per kilogram CO2 equivalent’ (kg CO2 eq) based on the most recent IPCC 100-year global warming potential [19]. The overall Coastal-Seawater scenario demonstrated a 3.5% reduction in climate change impact (Figure 1). Climate change is a relatively impactful category with European normalisation factors applied. This represents a significant improvement upon the Inland-Freshwater scenario. It worth noting that the use of seawater in washing the sugar beets alone enabled a 1.5% reduction in the climate change impact. The complete impact assessment results for beet washing only in both scenarios are indicated in Figure 2.
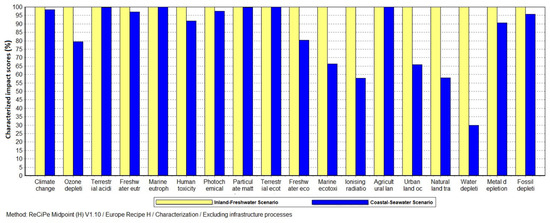
The water depletion describes the freshwater consumption in cubic metres (m3) of the system. Only groundwater and surface waters—i.e., freshwater sources—are considered. Seawater use in the Coastal-Seawater scenario displaces tap water and therefore does not contribute to water depletion. The impact assessment showed that the Coastal-Seawater scenario has 31.2% less impact on water depletion than the Inland-Freshwater scenario (Figure 1). In the beet washing stage, the use of seawater instead of freshwater reduced water depletion impact of the clean sugar beet by 70% (Figure 2).
The land use category factors in the type of land occupied by the process and inputs to the process. The units for occupation are in area and time occupied (m2*annum) whilst for transformation it is area (m2). Agricultural land occupation remained identical in the production of clean sugar beet and the endpoint production of bioethanol and its coproducts. The normalization shows that this is by far the most impactful of the selected categories for bioethanol production in Europe. Urban occupation was reduced in the Coastal-Seawater scenario by 34.2% for sugar beet washing (Figure 2) and 4.1% in the overall process (Figure 1). Natural land transformation was the most significantly reduced land use category with reductions in the beet washing and overall process of 41.9% (Figure 2) and 5.5% (Figure 1) respectively.
The Coastal-Seawater scenario showed a reduction of 4.2% in the fossil depletion impact category in both the washing stage and the overall process (Figure 1). The normalised results indicates that this is a significant category when results are calculated using European normalisation factors.
3. DiscCusrrent Insightsion
3.1. Water Depletion Impact and Other Benefits of Seawater Fermentation
Water in the Coastal-Seawater scenario is directly piped from the sea, a source that does not factor into WF as freshwater does. In the initial stage of water use in the process, sugar beet washing, use of seawater had 70% less water depletion impact of the clean sugar beet than the Inland-Freshwater scenario. Overall, the Coastal-Seawater scenario achieved a 31.2% net reduction in the water depletion impact compared to the Inland-Freshwater scenario. This demonstrates the potential of seawater usage in industrial scale bioethanol production to achieve a significant reduction in water depletion impact.
There is a high likelihood that through use of a seawater fermentation medium a high purity (97–99%) ethanol can be obtained for a fraction of the energetic cost associated with freshwater-based ethanol purification. Pure ethanol production would usually be done by a three-step extractive distillation of the fermentation broth: first distillation to 95% pure ethanol with 5% water, followed by extractive distillation requiring a solvent to increase the purity by water removal and finally distillation to remove the solvent. This process is highly energy intensive, as estimated by Lee and Pahl [20], where 50–80% of the overall process energy is required for producing highly pure ethanol. Addition of salts to the fermentation broth prior to extractive distillation has been suggested as a means to enhance ethanol recovery, reducing the number of stages to a single distillation [21]. In the coastal case, sodium chloride salt from the seawater medium is already dissolved in solution and thus may improve extraction efficiency and energy balance, which will be the focus of future research.
The scenarios were modelled with a single distillation stage yielding 95% pure bioethanol in the Inland-Freshwater scenario. The Coastal-Seawater scenario may have yielded a higher purity, due to the salt’s effect, and so produce higher value bioethanol. However, this requires support with practical data. The purity of the bioethanol produced has a direct link with economic value. Anhydrous ethanol is necessary for fuel use as water can induce corrosion and rust in engines.
3.2. Climate Change and CCS Technology
Bioenergy production has been suggested as an ideal industry for coupling with CCS technology [22], enabling a potential overall carbon negative process. The high purity stream of CO2 exiting the fermentation vessel can be easily collected for compression and storage, as compared to crude oil refineries which emit a range of gases and require a capture technology to filter emissions, far more processing and thus higher costs [2].
The CCS technology that is incorporated into conventional production, on which the Inland-Freshwater scenario in this study was modelled, tends to be short-term. Sequestration in products such as carbonated beverages delays CO2 release for the shelf life of the product. On the other hand, sequestration in the deep sea, such as geological injection, is a long term storage solution [23]. Offshore storage has been proposed as offshore continental shelves offer significant capacity which is necessary to meet 2050 climate goals [24]. Owing to the location of the Coastal-Seawater scenario, offshore oceanic injection is likely to be a feasible solution to store the CO2.
3.3. Coproduct Profiles and Economic Discussion
The investigated scenarios for bioethanol production yielded different coproduct profiles. Utilisation of waste streams (i.e., beet pulp and sea salt) adds value to the overall process and can thus potentially improve the economic viability of biofuels. A comprehensive techno-economic analysis to evaluate the financial performance can be the focus of future work. Both scenarios generate biogenic CO2 through fermentation that could be stored to increase the CCS ability of the bioethanol. However, there is a potential for more efficient CO2 storage in case of the Coastal-Seawater scenario. The CO2 generated during the fermentation at coastal locations can be stored safely and permanently in the deep sea (at 3000 m depth or more) as the CO2 becomes a heavy liquid that sinks to the ocean floor [25, 26][25][26].
Also, both scenarios produce beet pulp as animal feed but in case of the Coastal-Seawater scenario, beet pup is salted as a result of washing with seawater or by adding sea salt to the pulp following the juicing stage. In addition, if the process does not recycle the yeast for the next fermentation cycles, the salted yeast will be added to the pulp to produce a high value sea-salted animal feed. Salts are usually added to the feed, either by the retailer or by the consumer, as animals require the nutrients. Production of sea-salted animal feed in the coastal scenario comes without the expense of additive sea salt and can be presumably marketed at a greater value than unsalted feed.
In addition, the use of seawater in the Coastal-Seawater scenario enabled the additional production of distilled water and sea salt, neither of which are present in the Inland-Freshwater scenario. Sodium chloride, other minerals and trace elements are naturally contained in seawater. These remain dissolved through to the distillation stage at which point they are precipitated out, resulting in water and sea salt.
Also, addition of sea salt and distilled water to the coproduct profile further improves the economic situation in the Coastal-Seawater scenario. Originating from the sea, the water is desalinated in the process of distillation and so is converted from a free and abundant resource to a valuable product. By contrast the Inland-Freshwater scenario, which reflects current practices, treats this distilled water as waste even though it is usually required to be recycled in the fermentation medium to reduce the freshwater intake and the total WF of the process. However, reuse of the distilled water in the fermentation media is not desired because if used, additional minerals need to be added to the fermentation medium due to lower mineral content compared to tap water. Added revenue streams without significant additional costs make the Coastal-Seawater scenario the economically preferred scenario.
The value of water as a product dictates whether this element of the proposed coastal biorefinery balances the economic system. As a product of extractive distillation, the produced desalinated water would be non-potable. This is due to trace amounts of the solvent applied for ethanol recovery. Water utility companies identify a demand for non-potable water which lends a market value to this product. The purity of the water resulting from this process is not yet known. The water treatment required to achieve a potable product may be worth the cost.
Implementation of a biorefinery operating a seawater fermentation would be more beneficial to countries facing extreme water scarcity that have access to seawater. The value of desalinated water in these countries would be more economically favourable than in countries with plentiful freshwater resources. Desalination is an expensive method to produce water alone, but as part of the coproduct profile of bioethanol fuel production the economics may be more balanced.
3.4. Assumptions and Limitations
This study has been done under the assumption that the Coastal-Seawater scenario utilises a similar amount of electricity as in the Inland-Freshwater scenario. However, experimental investigation may indicate that the Coastal-Seawater scenario consumes less overall energy compared to the Inland-Freshwater scenario especially in beet washing and ethanol distillation steps and therefore, it may produce more surplus electricity. Also, this study assumed that both scenarios require the addition of similar amounts of sodium sulphate in the fermentation media, however, typical seawater (35% salinity) contains enough sodium (10.76 g/kg) and sulphate (2.71 g/kg) [27] and therefore addition of sodium sulphate could be eliminated in the Coastal-Seawater scenario. Anti-foam could be also eliminated or at least reduced in the Coastal-Seawater scenario because the high concentration of salt in seawater could work as an anti-foam [28]. In addition, unlike the Inland-Freshwater scenario, part of the distilled water produced in the Coastal-Seawater scenario can be reused in the system eliminating the need for external distilled water for cooling. Taking all these points into consideration for re-modelling the Coastal-Seawater scenario would lead to improved environmental and economic results.
Furthermore, bioethanol production rate and productivity were assumed to be equivalent in both scenarios. This is because the Coastal-Seawater model would ideally involve the use of marine yeast strain for fermentation in seawater-based medium. Marine yeast can produce bioethanol using seawater at almost the same rate as industrial terrestrial yeast using freshwater. The marine S. cerevisiae strain AZ65 recorded ethanol production at a rate of 1.62 g/L/h using seawater as compared to 1.65 g/L/h by terrestrial strain NCYC2592 using freshwater [13] and reached maximum ethanol productivity of 4.15 g/L/h using YPD-seawater medium and 2.46 g/L/h using Molasses-seawater medium [18]. A specific HPLC method for simultaneously measuring chloride, sugars, organic acids and alcohols in seawater samples was used in this research for accurate measurement of the ethanol and the remaining sugars [29]. The production rate and productivity of seawater-bioethanol can be improved by further optimisation of the fermentation parameters and improving the marine yeast strain.
On the other hand, the cost of construction of both the bioethanol plant and the oceanic CO2 injection site were not included in this study. This improved the comparability between the scenarios, if these structures have long enough lifetime that these capital costs can be paid back quickly. However, the capital investment in the two scenarios is likely to differ which can be addressed in future work.
3.5. Future Perspectives: Towards a Coastal Integrated Marine Biorefinery (CIMB) System
In this analysis the cultivation and harvesting of the feedstock subsystem produces identical results between the coastal and Inland-Freshwater scenarios. This has resulted in no change in agricultural land use between the two scenarios (Figure 1). The European normalisation factors, however, show that this is by far the most important of the selected categories.
Also, although the Coastal-Seawater scenario produces freshwater, its water depletion impact is not negative. This shows that the volume of freshwater produced does not offset that used in the production process. In the Coastal-Seawater scenario, seawater was applied to beet washing and the fermentation medium. However, the majority of water depletion takes place in the cultivation and harvesting.
This work was conducted to evaluate the environmental feasibility of the coastal setting and seawater use, with a view to then incorporate marine biomass (such as seaweed and marine microalgae) in the Coastal-Seawater system to reach a fully marine based biorefinery. This is predicted to greatly reduce environmental impact of water depletion and land use—agricultural land occupation to be specific. Further practical work will determine the processing requirements needed for marine biomass feedstock in a coastal biorefinery using seawater medium. However, new scenarios should be comprehensively analysed via environmental and social impact assessment using LCA and human health impact assessment using risk analysis to obtain comparable results [30, 31][30][31].
Marine biomass sources including macro- and microalgae have potential for use as feedstock. They do not require freshwater or land for cultivation and are therefore a desirable feedstock. A coastal location puts the production process in close proximity to marine resources. In addition to the access to seawater and marine biomass, the coastal location offers other potential benefits that have not been included in this study due to lack of data availability and research time. As the concept gains traction further integration with other technology systems could be explored. Another benefit of a coastal location is the ease of transportation by sea. The fuel product as well as the coproducts (water, salt, animal feed and LimeX) could be exported by freight shipping widening access to the fuel beyond its immediate area. Thus, a marine-based biorefinery in a coastal location is likely to improve both the sustainability and feasibility of bioethanol production [32-34][32][33][34] contributing to the global effort to achieve environmental targets.
Furthermore, coastal location improves accessibility to renewable power sources. In this analysis where electricity was necessary, such as in CO2 compression and beet pulp drying, the source of the electricity was from the grid which includes non-renewable sources. Direct connection to renewable sources, such as offshore windfarms and wave energy sites, could be integrated into the process to further improve the environmental impact towards net zero targets. This translates to reduced transport costs—both monetary and environmental—and may have further benefits.
References
1. United Nations, Paris Agreement, U. Nations, Editor. 2016, United Nations: Paris. p. 1-27.
2. IPCC, Special report on carbon dioxide capture and storage. 2005, Cambridge University Press, Cambridge.
3. GlobalCCSInstitute, Global Status of CCS Targeting Climate Change. 2019.
4. Pal, A., Blending of ethanol in gasoline: Impact on SI engine performance and emissions. International Journal of Thermal Technologies, 2014. 4(1): p. 1-5.
5. Al-Hasan, M., Effect of ethanol–unleaded gasoline blends on engine performance and exhaust emission. energy conversion and management, 2003. 44(9): p. 1547-1561.
6. Muñoz, M., et al., Bioethanol blending reduces nanoparticle, PAH, and alkyl-and nitro-PAH emissions and the genotoxic potential of exhaust from a gasoline direct injection flex-fuel vehicle. Environmental science & technology, 2016. 50(21): p. 11853-11861.
7. Transport, D.f. Fuelling a greener future: E10 petrol set for September 2021 launch. 2021 [cited 2021 04/03/2021]; Available from: https://www.gov.uk/government/news/fuelling-a-greener-future-e10-petrol-set-for-september-2021-launch.
8. Bailey, B.K., Performance of ethanol as a transportation fuel, in Handbook on Bioethanol. 2018, Routledge. p. 37-60.
9. Speidel, H.K. and I. Ahmed, Biodegradability characteristics of current and newly-developed alternative fuels. 1999, SAE Technical Paper.
10. Dias De Oliveira, M.E., B.E. Vaughan, and E.J. Rykiel, Ethanol as fuel: energy, carbon dioxide balances, and ecological footprint. BioScience, 2005. 55(7): p. 593-602.
11. European Union, Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the promotion of the use of energy from renewable sources (Text with EEA relevance.). Official Journal of the European Union, 2018. 328: p. 82-209.
12. Liu, X.V., S.K. Hoekman, and A. Broch, Potential water requirements of increased ethanol fuel in the USA. Energy, Sustainability and Society, 2017. 7(1): p. 18.
13. Zaky, A., et al., The establishment of a marine focused biorefinery for bioethanol production using seawater and a novel marine yeast strain. Scientific Reports, 2018. 8.
14. Gerbens-Leenes, W., A.Y. Hoekstra, and T.H. van der Meer, The water footprint of bioenergy. Proceedings of the National Academy of Sciences, 2009. 106(25): p. 10219.
15. Gerbens-Leenes, P., Green, blue and grey bioenergy water footprints, a comparison of feedstocks for bioenergy supply in 2040. Environmental Processes, 2018. 5(1): p. 167-180.
16. Domínguez de María, P., On the use of seawater as reaction media for large‐scale applications in biorefineries. ChemCatChem, 2013. 5(7): p. 1643-1648.
17. Zaky, A., et al., A New Isolation and Evaluation Method for Marine-Derived Yeast spp. with Potential Applications in Industrial Biotechnology. Journal of Microbiology and Biotechnology, 2016. 26(11): p. 1891-1907.
18. Zaky, A.S., et al., Improving the productivity of bioethanol production using marine yeast and seawater-based media. Biomass and Bioenergy, 2020. 139: p. 105615.
19. Stocker, T., et al., IPCC, 2013: climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. 2013.
20. Lee, F.M. and R.H. Pahl, Solvent screening study and conceptual extractive distillation process to produce anhydrous ethanol from fermentation broth. Industrial & Engineering Chemistry Process Design and Development, 1985. 24(1): p. 168-172.
21. Pinto, R., M. Wolf-Maciel, and L. Lintomen, Saline extractive distillation process for ethanol purification. Computers & Chemical Engineering, 2000. 24(2-7): p. 1689-1694.
22. Budinis, S., et al., An assessment of CCS costs, barriers and potential. Energy strategy reviews, 2018. 22: p. 61-81.
23. Laude, A., et al., CO2 capture and storage from a bioethanol plant: Carbon and energy footprint and economic assessment. International Journal of Greenhouse Gas Control, 2011. 5(5): p. 1220-1231.
24. Schrag, D.P., Storage of carbon dioxide in offshore sediments. Science, 2009. 325(5948): p. 1658-1659.
25. Rackley, S.A., Chapter 12 - Ocean Storage, in Carbon Capture and Storage, S.A. Rackley, Editor. 2010, Butterworth-Heinemann: Boston. p. 267-286.
26. Metz, B., et al., IPCC special report on carbon dioxide capture and storage. 2005: Cambridge: Cambridge University Press.
27. Morcos, S.A., Chemical Composition of Seawater and the Variation of Calcium and Alkalinity. ICES Journal of Marine Science, 1970. 33(2): p. 126-133.
28. Behera, M.R., et al., Foaming in Micellar Solutions: Effects of Surfactant, Salt, and Oil Concentrations. Industrial & Engineering Chemistry Research, 2014. 53(48): p. 18497-18507.
29. Zaky, A.S., et al., A new HPLC method for simultaneously measuring chloride, sugars, organic acids and alcohols in food samples. Journal of Food Composition and Analysis, 2017. 56: p. 25-33.
30. Papong, S., et al., Environmental life cycle assessment and social impacts of bioethanol production in Thailand. Journal of Cleaner Production, 2017. 157: p. 254-266.
31. Milazzo, M.F. and F. Spina, The use of the risk assessment in the life cycle assessment framework. Management of Environmental Quality: An International Journal, 2015. 26(3): p. 389-406.
32. Zaky, A.S., et al., Marine yeast isolation and industrial application. FEMS yeast research, 2014. 14(6): p. 813-825.
33. Greetham, D., et al., A brief review on bioethanol production using marine biomass, marine microorganism and seawater. Current Opinion in Green and Sustainable Chemistry, 2018. 14: p. 53-59.
34. Zaky, A.S., Marine fermentation, the sustainable approach for bioethanol production. EC Microbiology, 2017: p. 25-27.
References
- United Nations, Paris Agreement, U. Nations, Editor. 2016, United Nations: Paris. p. 1-27.
- IPCC, Special report on carbon dioxide capture and storage. 2005, Cambridge University Press, Cambridge.
- GlobalCCSInstitute, Global Status of CCS Targeting Climate Change. 2019.
- Pal, A., Blending of ethanol in gasoline: Impact on SI engine performance and emissions. International Journal of Thermal Technologies, 2014. 4(1): p. 1-5.
- Al-Hasan, M., Effect of ethanol–unleaded gasoline blends on engine performance and exhaust emission. energy conversion and management, 2003. 44(9): p. 1547-1561.
- Muñoz, M., et al., Bioethanol blending reduces nanoparticle, PAH, and alkyl-and nitro-PAH emissions and the genotoxic potential of exhaust from a gasoline direct injection flex-fuel vehicle. Environmental science & technology, 2016. 50(21): p. 11853-11861.
- Transport, D.f. Fuelling a greener future: E10 petrol set for September 2021 launch. 2021 [cited 2021 04/03/2021]; Available from: https://www.gov.uk/government/news/fuelling-a-greener-future-e10-petrol-set-for-september-2021-launch.
- Bailey, B.K., Performance of ethanol as a transportation fuel, in Handbook on Bioethanol. 2018, Routledge. p. 37-60.
- Speidel, H.K. and I. Ahmed, Biodegradability characteristics of current and newly-developed alternative fuels. 1999, SAE Technical Paper.
- Dias De Oliveira, M.E., B.E. Vaughan, and E.J. Rykiel, Ethanol as fuel: energy, carbon dioxide balances, and ecological footprint. BioScience, 2005. 55(7): p. 593-602.
- European Union, Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the promotion of the use of energy from renewable sources (Text with EEA relevance.). Official Journal of the European Union, 2018. 328: p. 82-209.
- Liu, X.V., S.K. Hoekman, and A. Broch, Potential water requirements of increased ethanol fuel in the USA. Energy, Sustainability and Society, 2017. 7(1): p. 18.
- Zaky, A., et al., The establishment of a marine focused biorefinery for bioethanol production using seawater and a novel marine yeast strain. Scientific Reports, 2018. 8.
- Gerbens-Leenes, W., A.Y. Hoekstra, and T.H. van der Meer, The water footprint of bioenergy. Proceedings of the National Academy of Sciences, 2009. 106(25): p. 10219.
- Gerbens-Leenes, P., Green, blue and grey bioenergy water footprints, a comparison of feedstocks for bioenergy supply in 2040. Environmental Processes, 2018. 5(1): p. 167-180.
- Domínguez de María, P., On the use of seawater as reaction media for large‐scale applications in biorefineries. ChemCatChem, 2013. 5(7): p. 1643-1648.
- Zaky, A., et al., A New Isolation and Evaluation Method for Marine-Derived Yeast spp. with Potential Applications in Industrial Biotechnology. Journal of Microbiology and Biotechnology, 2016. 26(11): p. 1891-1907.
- Zaky, A.S., et al., Improving the productivity of bioethanol production using marine yeast and seawater-based media. Biomass and Bioenergy, 2020. 139: p. 105615.
- Stocker, T., et al., IPCC, 2013: climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. 2013.
- Lee, F.M. and R.H. Pahl, Solvent screening study and conceptual extractive distillation process to produce anhydrous ethanol from fermentation broth. Industrial & Engineering Chemistry Process Design and Development, 1985. 24(1): p. 168-172.
- Pinto, R., M. Wolf-Maciel, and L. Lintomen, Saline extractive distillation process for ethanol purification. Computers & Chemical Engineering, 2000. 24(2-7): p. 1689-1694.
- Budinis, S., et al., An assessment of CCS costs, barriers and potential. Energy strategy reviews, 2018. 22: p. 61-81.
- Laude, A., et al., CO2 capture and storage from a bioethanol plant: Carbon and energy footprint and economic assessment. International Journal of Greenhouse Gas Control, 2011. 5(5): p. 1220-1231.
- Schrag, D.P., Storage of carbon dioxide in offshore sediments. Science, 2009. 325(5948): p. 1658-1659.
- Rackley, S.A., Chapter 12 - Ocean Storage, in Carbon Capture and Storage, S.A. Rackley, Editor. 2010, Butterworth-Heinemann: Boston. p. 267-286.
- Metz, B., et al., IPCC special report on carbon dioxide capture and storage. 2005: Cambridge: Cambridge University Press.
- Morcos, S.A., Chemical Composition of Seawater and the Variation of Calcium and Alkalinity. ICES Journal of Marine Science, 1970. 33(2): p. 126-133.
- Behera, M.R., et al., Foaming in Micellar Solutions: Effects of Surfactant, Salt, and Oil Concentrations. Industrial & Engineering Chemistry Research, 2014. 53(48): p. 18497-18507.
- Zaky, A.S., et al., A new HPLC method for simultaneously measuring chloride, sugars, organic acids and alcohols in food samples. Journal of Food Composition and Analysis, 2017. 56: p. 25-33.
- Papong, S., et al., Environmental life cycle assessment and social impacts of bioethanol production in Thailand. Journal of Cleaner Production, 2017. 157: p. 254-266.
- Milazzo, M.F. and F. Spina, The use of the risk assessment in the life cycle assessment framework. Management of Environmental Quality: An International Journal, 2015. 26(3): p. 389-406.
- Zaky, A.S., et al., Marine yeast isolation and industrial application. FEMS yeast research, 2014. 14(6): p. 813-825.
- Greetham, D., et al., A brief review on bioethanol production using marine biomass, marine microorganism and seawater. Current Opinion in Green and Sustainable Chemistry, 2018. 14: p. 53-59.
- Zaky, A.S., Marine fermentation, the sustainable approach for bioethanol production. EC Microbiology, 2017: p. 25-27.
