Human cytomegalovirus (HCMV) establishes both latent (where the virus is maintained in an absence of the production of infectious virions) and lytic (during primary infection and reactivation) phases of infection. The study of latency has been historically more difficult to study than that of lytic infection due to the relative quiescence of the virus in host cells and the limited cell types that support HCMV latency. With the advent of engineered HCMV viruses which express fluorescent tags that enable latently infected cells to be detected and isolated, it has been possible to analyse latently infected cells in much greater detail. In this article, we review studies which have used such systems to analyse and interrogate the latency associated proteome. These have led to the identification of cellular proteins and pathways important for latency and, consequently, potential therapeutic intervention drugs based on a greater understanding of latent infection.
- Human Cytomegalovirus
- Latency
- Proteome
1. IntroductioDefin
Human cytomegalovirus (HCMV) is a species-specific pathogen which is carried by approximately 60–80% of the population, depending on demographics [1]. Front-line anti-virals for HCMV are limited due to poor bioavailability and drug resistance. Additionally, currently available drugs target the lytic phase of infection rather than the latent phase (the phases of the lifecycle are described in detail below) [2][3]. Therefore, the need to target latently infected cells could be crucial, particularly in settings such as solid organ or stem cell transplantation where latent virus in the graft itself can be transferred to an immune-suppressed donor or a seropositive graft recipient’s own virus can reactivate.
2. HCMV Lytic and Latent Lifecycle
HCMV is a large double-stranded DNA virus with a protracted lytic lifecycle; up to 72 h in some cell types. This commences with immediate early (IE) gene expression, then early (E) gene expression and finally, late gene expression (L) [4]. The lytic lifecycle can take place in a wide range of terminally differentiated cell types, including fibroblasts, macrophages’ endothelial cells, resulting in full productive virus replication. The ability of the virus to establish a lifelong infection in the host is, at least in part, due to the ability of the virus to also be able to establish a latent infection during which time viral genome is carried in cells but IE gene expression is suppressed and infectious virions are not produced [5]. One established site of HCMV latency is in the cells of the myeloid lineage [5] where, in vivo, virus is carried in myeloid progenitors such as CD34+ progenitor cells and their derivative CD14+ monocytes. Then, as the cells differentiate along the myeloid lineage to terminally differentiated cell types such as macrophages or dendritic cells, the virus reactivates leading to the initiation of IE gene expression, the full temporal lytic gene cascade and ultimately, progeny virions [5].
3. Studyiiong the Proteome
During lytic infection, temporal changes in the viral proteome [4] as well as changes in the cell proteome mediated by lytic infection have been extensively analysed [4][6]. Such studies have been expanded upon, using for instance shRNA or CRISP-R libraries, to identify mechanisms by which viral-viral or viral-cellular protein–protein interactions regulate both cellular and viral gene expression during lytic infection [4][7]. Such high-throughput approaches to understanding HCMV lytic infection have been recently reviewed [7][8][9]. In this review, we discuss total proteomic analyses that have been carried out to interrogate cellular changes during HCMV latency.
4. Proteome Changes Induced by Expression of Latency-Associated Proteins in Isolation
Though unbiased proteomic screens are a powerful way to interrogate latency-associated changes in cellular proteins, some targets could possibly be missed, especially because viral latency-associated gene expression is known to be very low in the latently infected cells. One way to address this is to overexpress putative latency associated genes in isolation. Another advantage of this targeted approach is that a screen which identifies changes in total cellular proteins induced by latency will not, inherently, identify which viral gene product has caused these changes. Therefore, a number of studies have analysed changes in myeloid cells which over-express specific latency-associated proteins. To date, this has been carried out for two viral genes known to be expressed during latency by overexpressing them in THP1 myelomonocytic cells, a cell type known to support HCMV latency and reactivation [10][11][12]. In this entry, we have concentrated on changes to the cellular proteome. Many of these changes (summarised in Figure 1) have identified mechanisms by which latent infection modifies, transendothelial migration and avoidance of neutrophil killing.
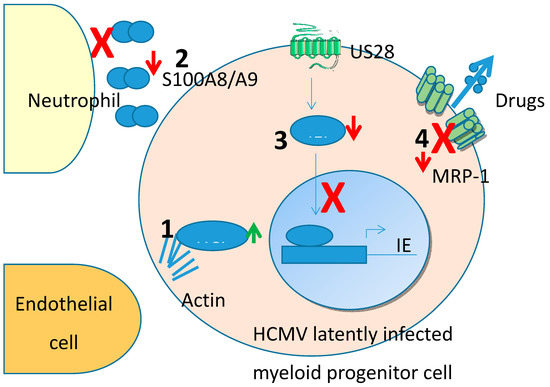
Figure 1.Human cytomegalovirus (HCellular protein changes identified from unbiased proteomic screens in MV) establishes either a latent (non-productive) or lytic (productive) infection depending upon cell type, cytokine milieu and the differentiation status of the infected cell. Undifferentiated cells, such as precursor cells of the myeloid lineage, support a latent infection whereas terminally differentiated cells, such as monocytes or dendritic cells are an environment conducive to reactivation and support a lytic infection. The mechanisms which regulate HCMV in either a latent or lytic infection have been the focus of intense investigation with a view to developing novel treatments for HCMV-associated disease which can have a heavy clinical burden after reactivation or primary infection in, especially, the immune compromised. To this end, a number of studies have been carried out in an unbiased manner to address global changes occurring within the latently infected cell to address the molecular changes associated with HCMV latency.
2. Introduction
Human cytomegalovirus (HCMV) is a species-specific pathogen which is carried by approximately 60–80% of the populatency. (1) HCLS1 is upregulated which regulates actin stabilisation anion, depending on demographics [1]. Front-line anti-virals for HCMV are limited due to poor bioavailability and drug resistance. Additionally, currently available drugs target the lytic phase of infection rather than the latent phase (the phases of the lifecycle are described in detail below) [2,3]. Therefore, the need to target latently infected cells could be crucial, particularly in settings such as solid organ or stem cell transplantation where latent virus in the graft itself can be transferred to an immune-suppressed donor or a seropositive graft recipient’s own virus can reactivate.
3. HCMV Lytic and Latent Lifecycle
HCMV is a large d,ouble-stranded DNA virus therefore,with a protracted lytic lifecycle; up to 72 h in some cell motility leading to enhanced endothelial cell attachment and transit. (2) S100A8/A9 are downregulated preventintypes. This commences with immediate early (IE) gene expression, then early (E) gene expression and finally, late gene expression (L) [4]. The lytic lifecycle can take place in a wide range of terminally differentiated cell types, including fibroblasts, macrophages’ endothelial cells, resulting in full productive virus replication. The ability of the virus to establish a lifelong infection in the host is, at least in part, due to the ability of the virus to also be able to establish a latent infection during which time viral genome is carried in cells but IE gene expression is suppressed and infectious virions are not produced [5]. One established site of HCMV latency is in the cells of the myeloid lineage [5] where, in vivo, virus is carried in myeloid progenitors such as CD34+ progenitor cells and their derivative CD14+ monocytes. Then, as the cells differentiate along the chemoattraction of neutromyeloid lineage to terminally differentiated cell types such as macrophages or dendritic cells, the virus reactivates leading to the initiation of IE gene expression, the full temporal lytic gene cascade and ultimately, progeny virions [5].
4. Studying the Proteome
During lytic infection, temporal chils which could otherwise target and kill latentlyanges in the viral proteome [4] as well as changes in the cell proteome mediated by lytic infection have been extensively analysed [4,36]. Such studies have been expanded upon, using for instance shRNA or CRISP-R libraries, to identify mechanisms by which viral-viral or viral-cellular protein–protein interactions regulate both cellular and viral gene expression during lytic infection [4,37]. Such high-throughput approaches to understanding HCMV lytic infected cells. (3) IFI16 is downregulateion have been recently reviewed [37–39]. In this review, we discuss total proteomic analyses that have been carried out to interrogate cellular changes during HCMV latency.
5. Proteome Changes Induced by Expression of Latency-Associated Proteins in Isolation
Though unbiased proteomic screens are by a powerful way to interrogate latency-associated changes in cellular protein US28 and prevents NFkB activation of the MIEP and therefore helps aid the maintenance ofs, some targets could possibly be missed, especially because viral latency-associated gene expression is known to be very low in the latently infected cells. One way to address this is to overexpress putative latency associated genes in isolation. Another advantage of this targeted approach is that a screen which identifies changes in total cellular proteins induced by latency will not, inherently, identify which viral gene product has caused these changes. Therefore, a number of studies have analysed changes in myeloid cells which over-express specific latency. (4) Drug transporter MRP-1 is downregulat-associated proteins. To date, this has been carried out for two viral genes known to be expressed during latency by latency associated protein UL138 which renders cells sensitive to drug-mediatedoverexpressing them in THP1 myelomonocytic cells, a cell type known to support HCMV latency and reactivation [7,46,47]. In this entry, we have concentrated on changes to the cellular proteome. Many of these changes (summarised in Figure 1) have identified mechanisms by which latent infection modifies, transendothelial migration and avoidance of neutrophil killing.

