Shoot apical meristems (SAM) are tissues that function as a site of continuous organogenesis, which indicates that a small pool of pluripotent stem cells replenishes into lateral organs. The coordination of intercellular and intracellular networks is essential for maintaining SAM structure and size and also leads to patterning and formation of lateral organs. Leaves initiate from the flanks of SAM and then develop into a flattened structure with variable sizes and forms. This process is mainly regulated by the transcriptional regulators and mechanical properties that modulate leaf development. Leaf initiation along with proper orientation is necessary for photosynthesis and thus vital for plant survival. Leaf development is controlled by different components such as hormones, transcription factors, miRNAs, small peptides, and epigenetic marks. Moreover, the adaxial/abaxial cell fate, lamina growth, and shape of margins are determined by certain regulatory mechanisms. The over-expression and repression of various factors responsible for leaf initiation, development, and shape have been previously studied in several mutants. However, in this review, we collectively discuss how these factors modulate leaf development in the context of leaf initiation, polarity establishment, leaf flattening and shape.
Introduction
Leaves are the primary organs responsible for photosynthesis and photoperception, and play a key role in plant growth. Their development starts from the shoot apical meristem (SAM), which have a central zone (CZ) that houses pluripotent cells, and a peripheral zone (PZ), responsible for the leaf initiation and their development into a flattened structure [
1]. In most plants, the leaf functions as a solar panel, where photosynthesis converts carbon dioxide and water into carbohydrates and oxygen [
2]. Leaves are an excellent example of learning how complex organs arise from a simple structure. All leaves are initiated by the recruitment of cells flanking the SAM as simple rod-like primordia, later on, to get their final shape. There are three principal axes in a leaf, along which intrinsic genetic programs control leaf cell division, differentiation, and expansion. However, leaf morphogenesis is strictly controlled not only by intrinsic genetic factors but also by hormonal factors. Numerous series of events demonstrate that plant hormones, mostly small and simple molecules, play crucial roles in plant growth and development [
3]. It was hypothesized how hormonal and genetic networks regulate leaf morphogenesis to enable the transformation of simple primordium into a complex organ with consistent shape and size, and to elaborate how these genetic networks generate plasticity in response to both endogenous and environmental signals. Since a deeper understanding of leaf development contributes to our overall comprehension of plant biology, this understanding can also be used to improve crop production. Therefore, it is important to unveil the molecular and hormonal regulation of leaf morphogenesis, including the initiation of leaf primordia, the determination of leaf axes, and the regulation of cell division in model plant
Arabidopsis thaliana.
1.1. Maintenance of Shoot Apical Meristems (SAM) and the Leaf Initiation Gene Network
The stem cells of plant meristems generate new organs and tissues throughout the life of the plant. The above-ground organs in plants are formed through SAM, whereas below-ground organs are created by root apical meristems (RAM). Beside these two apical meristems, various other types of meristems exist in plants, such as lateral meristems (e.g., axillary in the node of the leaves and flowers), intercalary meristems (at the base of the monocots leaf blade), and transient stem-like meristemoids (the precursor for guard cells) [
6,
7]. The SAM is a dome-shaped structure that comprises a reservoir of stem cells, provides cells that form the branches, leaves, and flowers of the plant, and also retains its own identity. The SAM is organized into several distinct cell layers and various zones [
8] (). The development and maintenance of SAM are crucial for determining the spatiotemporal arrangement (e.g., clockwise, anticlockwise, spiral and whorled) of aerial organs around the stem. The process of arranging different organs spatially is known as phyllotaxis, which is species and stage-dependent [
9,
10,
11].
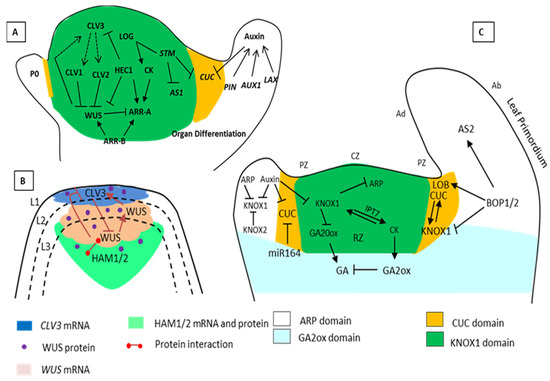
Figure 1. Schematic representation of Shoot Apical Meristems (SAM) maintenance by the various interacting genes. (A) The pluripotent stage and a specific number of cells in the SAM are controlled by the (WUS/CLV3) negative-feedback loop. CLAVATA3 (CLV3) is a ligand attached to CLV1 and CLV2 for the restriction of WUS. However, WUS activates CLV3 and works as a stem cell-promoting protein. STM activated the biosynthesis of CK through LONELY GUY (LOG). The STM and KNOX related genes also keep the stem cells undifferentiated by suppressing the expression of AS1 and gibberellic acid (GA) biosynthesis. STM also restricts the expression of the CUC gene due to negative regulators in a specific area. (B) The HAM and WUS-CLV3 loop. The regulatory loop requires CLV3, WUS, and HAM; the CLV3 negatively regulates the WUS expression, and the WUS protein moves from the organizing center to the active central zone (stem cells) to activate CLV3 expression. HAM1/2 is an interacting partner of WUS, and together with WUS protein suppresses the expression of CLV3 in the rib meristems. The expression zone of the CLV3 gene (blue), WUS gene (pink), HAM proteins (green) and the dot marks the WUS protein. (C) Regulatory networks that control leaf initiation. The cells in the SAM are arranged into layers L1, L2, and L3 and further into a distinct group of either tunica or corpus. According to the expression of genes, the SAM architecture is organized with the central zone (CZ), peripheral zone (PZ), organizing zone (OZ), and rib zone (RZ). During leaf initiation, auxin maxima repress the expression of the KNOX1 domains (gene) indeterminate meristem domains. KNOX1 maintains a high level of CK and low levels of GA in the meristem. In the ARP domain that has leaf identity, the leaf primordium separates from SAM by expression of boundary specific genes CUC and BOP regulates the petiole specification and polarity (positive and negative regulations are indicated by pointed and T-shaped arrows).
In
Arabidopsis thaliana, the SAM possesses three cell layers (L1–L3). An external two-cell layer forms tunica, where the L1 overlies L2. This layer divides by anticlinal cell division and grows in a two-dimensional fashion [
12]. An inner layer (L3) divides both periclinal and anticlinal cell divisions in a mostly random fashion, which is commonly called corpus. These three histogenic layers are responsible for producing different parts; the L1 layer divides and forms the entire shoot epidermis while the L2 layer produces the photosynthesizing cells of the sub-epidermis. The L3 layer forms the internal tissue, pericycle, and other corpus cells [
13].
The SAM layers are further subdivided into three functional domains or zones according to the function and division rate (). The three zones include the central zone (CZ), peripheral zone (PZ), and rib zone (RZ), which is present below the CZ. The CZ is mainly responsible for the maintenance of SAM. The CZ contains both tunica and corpus cells in which the stem cells are present, and below the CZ is the organizing center (OC) [
14]. The tunica and corpus cells of the CZ are symplasmically interconnected through the plasmodesmata [
15,
16]. Any alteration in the intercellular signals through the plasmodesmata affects normal growth and development [
17].