Almond (Prunus dulcis(Mill.) D. A. Webb, Prunus amygdalus Batch, or Amygdalus communis L.) constitutes the most produced nut worldwide, thanks to its exceptional nutritional composition, including low sugar content, high levels of proteins, unsaturated fatty acids, vitamins, and minerals, as well as health-enhancing phytochemicals.
- almond skins
- almond shells
- almond hulls
- blanching water
- waste management
- circular economy
- food fortification
- allergens
- sustainability
1. Introduction
Figure 1 shows the common workflow associated with the industrial procedure of almond production. As it is shown, the almond trees are subjected to pruning, thus promoting the generation of branches and leaves that are normally discarded and little attention has been paid to the valorization of these matrices. When drupes ripen, they are harvested and subjected to processing to obtain the almond kernels, or almond meat, which are the major product commercially exploited, involving the generation of the classically known as almond by-products [1][6]. Harvested drupes enter a first stage of hulling, in which the external coating is removed, forming almond hulls that accounts for the 52% of total produced mass; then, shelled almonds are subjected to shell removal, obtaining the coated almond kernels separated from shells, that represent the 33% of the total fruit. Finally, kernels, which constitutes the 11% of the original almond fruits, are blanched in order to eliminate the skins (which represents the 4%) by a treatment with hot water followed by a final peeling step [2][3][7,8]. As a result, four main by-products have gained much attention in the sector of almond production ( Figure 1 ): almond hulls (AHs), almond shells (ASHs), almond skins (ASKs), and blanching water (BW). On the other hand, almond kernels (AKs) are considered as a dense nut with high protein (16%) and lipid contents, the latter representing the 50% of AKs, mostly containing monounsaturated fatty acids (MUFAs, 32.2%), polyunsaturated fatty acids (PUFAs, 12.2%), and a low remaining content of saturated fatty acids (SFAs, <4%) [3][8]. The rest of nutrients associated to AKs, are represented by a polysaccharidic fraction, containing high fiber and starch proportions, together with a low quantity of simple sugars, and multiple minerals, and vitamins, being an exceptional source of vitamin E (in the form of α-tocopherol) [4][5][9,10].
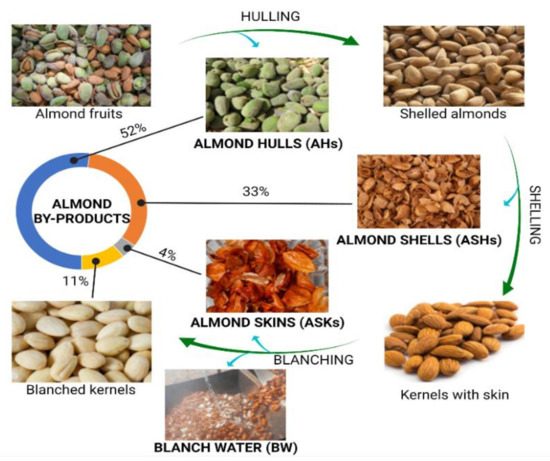
Almond hulls (AHs) refer to the fruit mesocarp of almonds, being the heaviest by-product formed during almond production. They are not destined to nutritional purposes because of their dryness (8–20% of moisture content), leathery texture, and astringency, due to their prolonged exposure to environmental threats [6][2]. Almond shells (ASHs) constitute the lignocellulosic layer found in the thick endocarp of almond fruits. Due to their woody nature and poor industrial applicability, ASHs are eliminated by incineration or indiscriminate dumped [7][11]. ASKs is the term used to refer to seedcoat, and are the most explored almond by-product in terms of their content of bioactive compounds, since they are often consumed with the kernel on which they play a paramount role in the antioxidant and antimicrobial defense [6][2]. BW has been also recently characterized in terms of the bioactive compounds extracted from the kernel during blanching, being legally considered as an industrial waste that should be specially disposed [8][12]. Among the different applications proposed for the revalorization of almond by-products, such as livestock feeding [9][13], biofuel production [10][14], and active carbon formulation [11][15], a nutritional perspective has been carried out with the aim of characterizing their phytochemical composition, in terms of bioactive compounds production, exploring their suitability as sources of functional ingredients to be applied in the food, pharmaceutical, and cosmetic industries.
Among the different bioactive compounds (BCs) found in almond by-products most reports have been focused on phenolic compounds, including mainly phenolic acids and flavonoids, polysaccharides, terpenoids, and fatty acids ( Figure 2 ). Thus, a recent review by Prgomet et al. [12][4] widely reported the characterization of almond by-products as a promising source of bioactive compounds, especially phenolic compounds. In addition, these authors review the functional properties of such compounds on the prevention of degenerative diseases. Consequently, an in-depth description of bioactive compounds of almond by-products is required to provide insight about the potential uses of these resources in the field of natural product research.
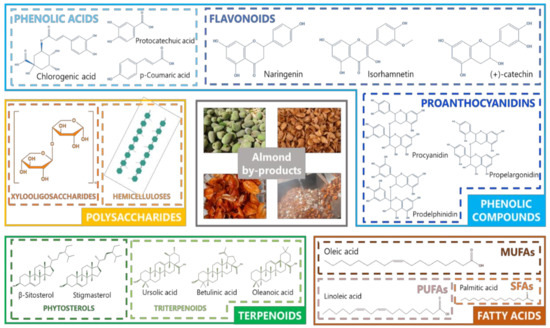
There is a wide evidence regarding the potential benefits of almond by-products as promising sources of bioactive compounds. The effectiveness of such compounds from almond residues as antioxidant, anti-inflammatory, anticancer, antimicrobial, antiviral, prebiotic, cardioprotective, antidiabetic, and anti-obesity agents have been largely assessed as a result of countless in vitro and in vivo studies, as well as by different interventional clinical trials in humans [13][14][35,36]. Thus, almonds together with their by-products can be used as active ingredients for the prevention of chronic diseases, such as cancer, diabetes, and cardiovascular diseases. Due to their precedence from an edible nut, almond by-products can be then applied for the production of new functional foods and nutraceuticals [12][4]. In this review, a current perspective on the exploitation of almond by-products as sources of bioactive compounds will be proposed, with a special focus on the extraction technologies applied to that end, as well as the description of already-established strategies committed to the fortification of different foods, together with the limitations on the applicability of these by-products. Finally, in order to meet the current global requirements of the food industry concerning environmental consciousness, the exploitation of almond by-products will be incorporated into a sustainability context, exhibiting recent and future strategies aiming at the establishment of a circular economy around the almond industry.
2. Food Fortification Using Almond By-Products
The incorporation of foods with almond shell, hull and skin or their extracts is shown in Table 1 . Recent studies have explored both the use of almond by-products as feedstock to produce foods of animal origin and their incorporation as ingredients in foods. Regarding the incorporation into animal feedstock, a recent study with milk cows indicated that AHs (7–20% in feeding) caused a significant effect in the content of milk fat and protein contents [9][13]. Linear correlations were obtained between these macronutrients and the level of AHs in feeding: fat content and protein content decreased with the increasing levels of AHs in cow feeding, thus suggesting an enhancing nutritional potential associated with these almond by-products. These effects were attributed to the metabolism of carbohydrates and proteins in the rumen, according to the authors. A related experiment with AHs reported contrasting results in terms of milk composition [15][82]. In this case, the incorporation of AHs did not affect fat, protein, or lactose content. Moreover, no effect in terms of fatty acids were observed between the milk samples obtained from cow in control and almond hull diet. As a result, the influence of AHs as feedstock remains unclear and further investigations are required on this concern.
Table 1. Fortification of food products with almond by-products.
| Almond By-Products | Administration | Fortified Food | Nutritional and Technological Effects | References |
|---|---|---|---|---|
| AHs | 7–20% in cow feedstock | Milk | Increased fat content, reduced protein content, no effect in lactose and total solids | [9][13] |
| 4.0 kg of dry matter/day in cow feedstock | Milk | No effect in fat, protein and lactose contents or fatty acids | [15][82] | |
| 7.5 and 15% in laying hens feedstock | Eggs | No effect in egg quality | [16][83] | |
| AHs and ASHs | Bed of feedstock | Edible larvae | Increased harvest weight, harvest yield and calcium content | [17][84] |
| ASHs | 3–15.3% alkali extract | Biscuit | Increased TDF, SDF and IDF, a* value, and hardness; reduced L* and b* values, sensory scores for color; no effect in sensory scores for flavor, crispness, mouthfeel, hardness, and overall acceptance | [18][85] |
| ASKs | (30–100 g/kg) | Wheat flour dough | Increased water absorption, dough stability, tenacity/extensibility ratio, L* and a* value; reduced softening index, deformation energy, b* value; no effect in dough development time | [19][50] |
Besides feedstock, almond by-products have been recently explored in the basis of ingredients for the fortification of food products. In this way, the potential fortification of eggs with AHs was studied in a recent study [16][83]. In this case, AHs were added to the feeding of laying hens at two levels: 7.5 and 15%. According to these authors, no significant effects were reported in terms of egg quality (percentages of yolk, albumen, and shell, Haugh unit, specific gravity, and egg size). Interestingly, the authors reported that hens in the supplemented group displayed a reduction in fat and lean mass in relation to animals in non-supplemented group.
Another interesting experiment was recently carried out to effect of AHs and ASHs as a bed of feedstock in the production of edible black soldier fly ( Hermetia illucens L. ) larvae [17][84]. In this case, the use of almond by-products bed in combination with aeration increased the content of calcium by 18% in the insects. Additionally, the group of larvae reared in almond by-products beds with high aeration rate in the bioreactor had higher harvest weight and yield (three and five times higher, respectively) than those produced using a bed with low aeration rate.
These studies indicate that almond by-products, mostly hulls and skins, can be a seen as a multicomponent source of nutritionally relevant compounds for food fortification for the incorporation either in animal feeding or in the use as food ingredient in processed foods. However, more studies are necessary to improve the knowledge about the factors limiting the fortification of food with almond by-products, especially in animal feedstock studies.
3. Limitations on the Applicability of Almond By-Products
According to the literature, the incidence of nut allergy in the general population is 1%. Almonds are nuts that are commonly consumed worldwide, and their consumption constitutes a potential allergen risk [20][86]. Indeed, the allergens from almonds are the third most common reported nuts allergens in the United States of America behind cashew nut and walnut [21][87]. Thus, numerous food allergens have been characterized from eight native almonds on the basis of their biochemical functions ( Table 2 ). Amongst them, there are four almond-derived allergens officially reported as food allergens, according to the World Health Organization (WHO)–International Union of Immunologist Societies (IUIS) list, namely: Pru du 3, Pru du 4, Pru du 5, and Pru du 6 [22][23][24][25][26][88,89,90,91,92].
Table 2. Almond-derived food allergens and their clinical effects.
| Allergens | Mw | Biochemical Functions | Effects on Food Processing | Clinical Effects |
|---|---|---|---|---|
| Pru du (γ-conglutin) | 45 kDa | Vicillin storage protein | n.d. | Unclear symptoms |
| Pru du 1 | 17 kDa | Protection against pathogens and environmental stresses | Wet heat protection | Reduction of immunoglobulin E (IgE)-mediated reactivity. Mild immune reaction |
| Pru du 2 | 23–27 kDa | Protection against pathogens and osmotic stresses | Heat and pH protection | Potent immunogenicity |
| Pru du 2S | 12 kDa | Nut storage protein | Heat resistance | Unclear symptoms |
| Pru du 3 | 9 kDa | Lipid transfer protein | Heat and pH protection | Systemic and life-threatening symptoms |
| Pru du 4 | 14 kDa | Actin-binding protein | Heat dissipation | Mild immune response |
| Pru du 5 | 10 kDa | Involvement in protein synthesis | Thermal stability | IgE-mediated allergic reactions |
| Pru du 6 (amandin) | 360 kDa | Storage protein | Thermal stability | Severe IgE-mediated allergic reactions |
In general, almond-mediated allergies are usually associated with poplar pollen allergies from other fruits of Rosaceae family members. In most of the cases, immune reactions are mild with a prominent clinical manifestation related to oral allergy syndrome [27][28][93,94]. Pru du 6, also known as amandin, is one of the firstly described allergens from almonds, as well as the major almond allergen, accounting for approximately 65% of total almond proteins, so it can induce a severe immune reaction upon ingestion [29][95]. In 2009, Pru du 3 (non-specific lipid transfer protein) was added into the WHO-IUIS allergen database [30][96]. In 2006, Pru du 4 was classified as a food allergen and added into the WHO-IUIS allergen database, as well, after the conduction of a study in which more than 40% of participants developed clinical symptoms derived from this allergen consumption [25][91]. In short, almond is largely used by the food industry due to its flavor, nutrients, and numerous health benefits despite the development of several allergic reactions. In this sense, little efforts have been made on the study and characterization of these allergens, in comparison with other nut-derived allergens, such as those of peanuts and other tree nuts. Therefore, there is an urgent need to explore the potential allergens proceeding from almond, along with their proper nomenclature and the definition of their structure-function relationships.
As essential economical resources of several regions worldwide, including California and Mediterranean countries, almond production is constantly subjected to several studies focused on the mycotoxin contamination, being one of the leading problems behind production and quality losses in this sector. The most prominent fungal contaminations reported on almonds are those caused by different strains belonging to Aspergillus , Eurotium , Penicillium , and Rhizopus . Much attention has been paid to both Eurotium spp. and Aspergillus spp. contaminations, causing a severe negative impact on all the stages of almond production cycle, from field harvesting to storage and market production [31][32][97,98]. Thus, among the several problems associated with fungal contaminations, aflatoxins produced by different Aspergillus species constitute one of the major threats to almond production, which may proliferate under insufficient storage and handling conditions [33][99].
Aflatoxins are difuranocoumarin isomers, named as AFB1, AFB2, AFG1 and AFG2, differentiated by their fluorescent properties [34][100]. These compounds may cause severe toxicity and carcinogenicity in numerous animals as well as in humans, in which their consumption leads to a systemic symptomatology collectively known as aflatoxicoses, characterized by hemorrhages, acute liver damage, edema, digestive disorders, malabsorption, and, eventually, death [35][101]. Hence, fungal contamination is a major problem in almond processing. Since the full eradication of fungal contamination is a hard goal to achieve, understanding the occurrence and diversity of fungal population and their mycotoxigenic potential, as well as improving the environmental and storage conditions, will greatly contribute to reduce the risk of contamination.
4. Sustainability and Future Perspectives for Almond Revalorization
As stated throughout this review, almond by-products can be incorporated in a wide range of foods because of their nutritional and phytochemical composition. Almond intensification ( Figure 3 ) provides a wide range of products, mainly focused on the food and cosmetic industries, including kernels, milk, oil, and flour. In the same way by-products have been exploited by means of different applications. Intense efforts are being developed with the aim of achieving a sustainable exploitation of the great amounts of waste generated during almond production, in order to establish a solid circular economy system around this sector.
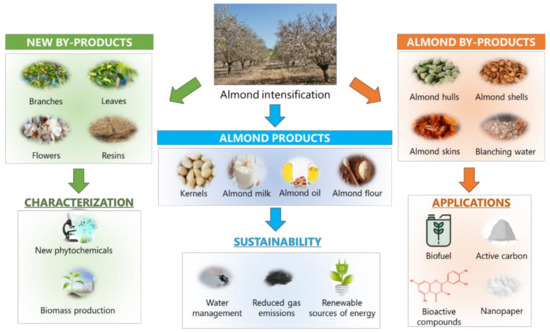
Besides the multifaceted applications of almond by-products, other strategies should be taken into account for the accomplishment of the sustainable goals proposed for this industry. Given the fact that the bioactive compounds from almond and its by-products are highly influenced by environmental conditions, rational approaches should be developed to increase the biosynthesis of these compounds. With the aim of promoting an environmental-friendly stimulation of bioactive compounds production, different authors have already revealed that irrigation regime plays a dual role on almond production, since water constitutes the main resource employed during almond production, accounting for the 45.8% of the environmental impact associated with this procedure [36][3]: on one hand, sustained deficit irrigation constitutes a paramount strategy to ensure a proper water management in wide areas involved in almond production, as it the case of semi-arid Mediterranean regions [37][117]; on the other hand, such limited water regime may drive to an improvement of physical and sensory parameters of almond fruits, as well as increasing the biosynthesis of bioactive compounds ( especially naringenin 7- O -glucoside and isorhamnetin-3- O -rutinoside), as already reported for AHs, which exhibited an increase in the irrigation-mediated production of phenolic compounds, and ASKs, mostly influenced by the agro-climatic conditions [38][20]. As a result, water management has been revealed as a critical factor related to the sustainability of almond production, with a higher significance in arid regions, which represent the most prevalent production areas worldwide.
In addition to water management, almond production must face additional issues concerning sustainable intensification, as it the case of greenhouse gases (GHG) emission. In detail, GHG production has been estimated, indicating that the production of 1 kg of raw almond and by-products provokes the emission of 1.6 kg of carbon dioxide equivalents (CO 2eq ) [39][118]. In order to counter almond production-associated pollution, almond by-products, mostly ASHs, have been reported as biobased heat and energy sources, and recently suggested to be subjected to scaled-up bioreactors for its large-scale production [40][119]. In fact, the feasibility of such application has been already assessed, showing a carbon conversion efficiency of 75%, as recently proved by Kaur et al. (2020) [10][14]. AHs have been also regarded as a potential source of biofuels, through the recently coined concept of “almond refinery”, in which hulls are subjected to hydrothermal treatment to simultaneously synthesize biofuels and valuable compounds [41][120].
On top of the well-established almond by-products, largely reported for their valuable applications, the sustainability of almond production can be still improved and optimized. There are some under-explored residues, produced during almond production, which require much attention regarding the exploitation as profitable resources. In this way, as it occurred with ASHs, the lignocellulosic profile of almond tree bark and branches from pruning have been recently assessed as effective natural sources of ACs, promoting the removal of highly contaminant synthetic dyes from wastewater [11][15], as well as a source of biofuel via wet torrefaction [42][125]. Moreover, almond tree leaves and flowers are another side by-products obtained from pruning that should be also subjected to re-valorization, especially in terms of bioactive compounds production, as it occurs with olive tree leaves, and biomass collection to produce green energy.
