Two-pore domain potassium (K2P) channels are the latest potassium channels family to be described. Since the discovery of the first member of the TWIK subfamily, the 15 known K2P channels cloned to date have been grouped into six different subfamilies: TIWK; TREK; TASK; THIK; TRESK; and TALK. In mammals, K2P channels are expressed in both nervous and non-nervous tissue, and their distribution varies widely from the almost ubiquitous expression of TWIK to the weak pancreatic expression of TALK.
- K2P channels
- TWIK
- TASK
- TREK
- heart
1. Introduction
Two-pore domain potassium (K2P) channels are the latest potassium channels family to be described. Since the discovery of the first member of the TWIK subfamily [1], the 15 known K2P channels cloned to date have been grouped into six different subfamilies: TIWK; TREK; TASK; THIK; TRESK; and TALK [2,3,4,5][2][3][4][5]. In mammals, K2P channels are expressed in both nervous and non-nervous tissue, and their distribution varies widely from the almost ubiquitous expression of TWIK to the weak pancreatic expression of TALK [6]. In the nervous system, K2P channels have been identified at both the central [1,7,8,9,10,11,12,13,14,15][1][7][8][9][10][11][12][13][14][15] and peripheral level, including the somatic [16,17,18,19,20][16][17][18][19][20] and autonomic nervous systems, where our group has contributed significantly [21,22,23,24][21][22][23][24].
Although the first K2P channel was not cloned until 1996, a barium-sensitive potassium current had already been identified in ventricular cardiomyocytes in 1993 using the Patch-clamp technique, and it had been proposed to be involved in the duration of the action potential (AP) plateau in guinea pigs [25]. Previously, a channel sensitive to changes in negative pressure, arachidonic acid (AA) and intracellular pH (pHi) had been demonstrated in rat cardiomyocytes [26], and even earlier, a potassium current sensitive to AA and mechanical stimuli had been identified in rat cardiomyocytes [27]. Significantly, the characteristics of these currents were consistent with the properties described later for some TREK channels of the K2P subfamily. Subsequently, several K2P channels were detected in cardiac tissue by quantitative real-time polymerase chain reaction (qRT-PCR), including TASK-1, TASK-3, TWIK-1, TREK-1, THIK, TALK-2 and TREK-2 [28,29,30,31,32,33][28][29][30][31][32][33]. However, in cardiomyocytes and nodal cells, the TASK, TWIK and TREK subfamilies were the most strongly expressed in mammals [34]. As such, we can now define where TASK, TWIK and TREK channels are expressed in the mammalian heart ( Figure 1 ), although the expression of other K2P subfamilies in the heart seems to depend on the species analyzed. Here, we aim to bring together the most relevant information regarding cardiac TWIK, TASK and TREK channels, focusing on their putative roles in cardiac physiology and their involvement in coronary pathologies.
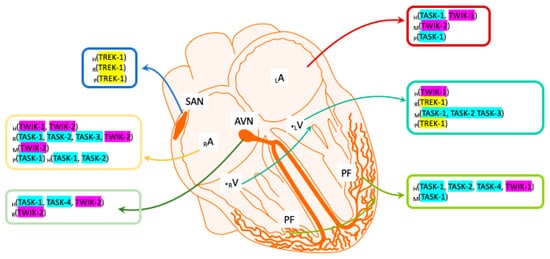
2. Two-Pore Domain in a Weak Inward Rectifying K+ Channel (TWIK)
TWIK-1 was the first human K2P channel cloned [1], and it is now considered to be part of the “tandem of pore domains in a weak inward rectifying K+ channel” (TWIK) subfamily that is made up of TWIK-1 (KCNK1) , TWIK-2 (KCNK6) and TIWK-3 (KCNK7). TWIK channels are sensitive to changes in pHi, barium and quinine [35,36,37][35][36][37]. It was initially reported that TWIK-1 (also called cTBAK-1) is expressed more strongly in human ventricular myocytes than in atrial myocytes based on RT-PCR and Northern blot analysis [1,38][1][38]. However, TWIK-1 was later reported to be more intensely expressed in the atrium than in the ventricle [39[39][40][41],40,41], and it also seems to be expressed strongly in Purkinje fibers [42]. In murids, very strong expression of TWIK-1 was found in the whole rat heart, as confirmed by single-cell RT-PCR of both atrial and ventricular myocytes [29,43][29][43]. It was also shown that TWIK-2 is very abundant in the atrium and human atrioventricular node (AVN) [28]. Although this channel was thought to be expressed relatively homogeneously [37], it was later confirmed that TWIK-2 channels are more prominently expressed in the right atrium of both the human and rat heart [28], whereas no differences were observed in mouse ventricular or atrial cells [44].
Since TWIK mRNA is distributed widely in cardiac tissues, there is considerable information indicating that TWIK channels fulfil an important role in the physiology of the heart. In fact, it was proposed that TWIK channels might contribute to the heterogeneous cardiac inward rectifier potassium current named I K1 [38]. This current is mainly carried by Kir2.1 Kir2.2 channels [45[45][46][47],46,47], and it plays an important role in both the stabilization of the resting membrane potential (RMP) and in sculpturing the final phase of APs in muscle cells. Atrial fibrillation (AF) is one of the most widely studied heart disorders [48,49,50,51,52][48][49][50][51][52] and one of the most serious cardiovascular illness, clearly associated with the risk of heart failure [53]. The symptoms of AF include rapid and irregular palpitations, as well as fatigue and chest pain, with sinus irregularity usually underlying this disease. In humans, alterations to the expression of TWIK can contribute to the initiation or perpetuation of AF [40]. Specifically, reduced atrial TWIK-1 expression may be associated with chronic AF [39]. Alternatively, pathological, subphysiological K+ concentrations, known as hypokalemia, have also been related to AF, and TWIK-1 channels contribute to the stabilization of cardiomyocyte excitability under such conditions [54,55][54][55]. TWIK-2, another member of the TWIK subfamily, is very abundant in the atrium and human AVN, and since it is very sensitive to the antiarrhythmic drug dronedarone, it is thought to be a good target for the treatment of AF [28,56][28][56]. Finally, in patients with Brugada syndrome, a hereditary arrhythmic condition that causes sudden death, TWIK-1 is expressed strongly in Purkinje fibers [57], suggesting a putative role for TWIK channels is this pathological condition.
3. TWIK-Related Acid-Sensitive K+ Channels (TASK)
The TWIK-related acid-sensitive K+ channels (TASK) K2P subfamily is comprised of the TASK-1 (KCNK3), TASK-2 (KCNK5) , TASK-3 (KCNK9), TASK-4 (KCN17) and TASK-5 (KCNK15) channels. TASK channels are extremely sensitive to pHe (extracellular pH) changes in the physiological range (6.0 to 7.8) and to limited O 2 availability (hypoxia) [58,59,60,61][58][59][60][61]. Some members of the TASK subfamily are strongly expressed in rodent cardiac tissue relative to other organs such as the kidney or lungs, and TASK-1 is the K2P channel most strongly expressed in the rodent heart [58,62,63][58][62][63]. In cDNA libraries, moderate TASK expression was found in the mouse heart, yet in situ hybridization revealed strong TASK expression, especially in the atrium [58]. Broad expression of TASK mRNA was detected in the mouse heart in Northern blots [63], and strong right atrial expression was confirmed in the rat [28,29,62][28][29][62], with TASK subfamily members also expressed in the human heart [58].
Like TASK-1, TASK-2 and TASK-3 are also weakly expressed in the rat heart when assessed by RT-PCR [28[28][29],29], and they are poorly expressed in mouse ventricular myocytes [30]. By contrast, TASK-2 channels are strongly expressed in the right atrium in humans, and they are moderately expressed by Purkinje fibers [42]. Likewise, strong TASK-4 expression has been detected by qRT-PCR in human Purkinje fibers and in the AVN [68][64]. Finally, TASK-5 was also detected in Northern blots of human heart tissue [59]. A specific TASK-1/TASK-3 blocker (the aromatic carbonamide, A293) produced a clear decrease in the macroscopic outward current in rat cardiomyocytes in response to steps from positive potentials to −40 mV, as detected in two-electrode voltage-clamp recordings [29]. The effect of A293 indicated functional expression of TASK-1 channels in heart tissue [29,30[29][30][44],44], and similar results were found in human right atrial cells in whole-cell patch clamp experiments [34].
The expression of mouse and human TASK-1 channels in heterologous system (COS, Xenopus oocytes) and their study using the Patch-clamp technique showed a clear TASK-1-like kinetic response to changes in pHe, with moderate sensitivity to Zn 2+ , quinidine and barium inhibition [43,61,62,66][43][61][62][65], in conjunction with insensitivity to changes in pHi and internal Ca 2+ [58]. Both expression and functional data are clear indicators that TASK channels participate in cardiac physiology. Hence, TASK-1 channels could play an important role in shortening the length of APs, particularly as genetic (TASK -/- ) or pharmacological ablation elongates AP duration (APD) in rodent [29] and human [30,34][30][34]. Furthermore, electrophysiological studies in Langendorff-perfused hearts showed that fibers from TASK-1 -/- mice had longer APs and a higher heart rate (HR) than TASK-1 +/+ mice. In these conditions, no differences were seen in the P-wave and QRS duration, although there were clear differences in the QT interval that were enhanced in the knock-out (KO) animals [44]. Notwithstanding, a clear increase in both the QRS complex and APD was reported inTASK-1 -/- animals in vivo, yet not in the HR [30]. Since a substantial increase in the QT interval cannot be fully explained by changes in the APD in TASK-1 -/- mice, TASK-1 channels could influence the conductive capacity of heart tissues during development. Moreover, it has been speculated that the neuronal conduction system (composed of the His bundle, the branches and a conduction network of Purkinje fibers) in TASK-1 -/- animals could contribute to the lengthening of the QT interval [67,69][66][67]. In any case, it appears that TASK-1 channels can modulate the APD in human myocytes [66][65].
AF is the most common sustained arrhythmia [70][68], and patients with paroxysmal atrial fibrillation (pAF) and chronic atrial fibrillation (cAF) strongly overexpress the TASK-1 protein in the right atrium. In myocytes, TASK-1 channels contribute to AP repolarization and to background currents [64,66][69][65]. Since atrial myocyte AP shortening is the main mechanism underlying AF, it is reasonable to think that the overexpression of TASK channels in the atrium could trigger this cardiac alteration. The antiarrhythmic effect of TASK-1 is suppressed in pigs with AF [64][69], suggesting that these channels may constitute an important therapeutic target to stabilize the HR [39]. Conversely, it has also been assumed that TASK-1 downregulation is the basis of heart failure [70][68]. Therefore, it seems that as in other pathologies and for other K2P channels [71][70], the dysregulation (up or down) of TASK is correlated with AF.
4. TWIK-Related K+ Channels (TREK)
The TWIK-related K+ channel (TREK) subfamily includes the TREK-1 (KCNK2), TREK-2 (KCNK10) and TRAAK (KCNK4) channels [2,14,73][2][14][71]. TREK channels are especially sensitive to AA, changes in pHi and mechanical stimuli [15[15][72][73][74][75][76][77][78],74,75,76,77,78,79,80], as well as changes in temperature and membrane stretching [81,82][79][80]. In non-neural rodent cardiac preparations that mainly include cardiomyocytes and fibroblasts, the presence of TREK-1 channels has been largely confirmed by real-time RT-PCR and immunohistochemistry [28,33,74,83,84][28][33][72][81][82]. TREK-1 expression is quite heterogeneous, and in adult rats, there is less TREK-1 in epicardial than in endocardial myocytes [85,86][83][84]. In mice, TREK-1 channels are preferentially expressed in the ventricle [44], one of the most strongly expressed K2P channels in ventricular tissue [30]. However, in other animals such as pigs, a more uniform pattern of expression for TREK-1 is evident in atrial and ventricular myocytes [70][68]. The expression of TREK-1 channels in the adult human heart seems to be less important than in mouse [33], although there is intense TREK-1 mRNA in atrial appendages and the sinoatrial node (SAN) regions [34] . In fact, TREK-1 expression has been reported in SAN cells in the rat [28,83,86,87][28][81][84][85], mouse [88][86] and pig [70][68].
TREK-2 and TRAAK channels are considered to play a very limited role in the physiology of the heart [89,90][87][88]. Moderate TREK-2 and weak TRAAK expression has been reported in the rat heart by RT-PCR [28]. Unlike TREK-1 and TREK-2, the presence of TRAAK channels has not been confirmed in the mouse heart [29[29][89],91], although some splice variants of TRAAK channels have been located in human heart tissue [92][90]. As early as 1993, a possible role for a TREK-compatible activity in cardiac physiology was suggested [25]. Before TREK-1 was first cloned in 1996 [84][82], a new K+ conductance was found in neonatal rat atrial cells that responded to AA and changes in pHi. In inside-out patch-clamp experiments, the activity of these channels augments in the presence of AA and when the pHi is sequentially modified from 7.2 to 6.0 [27]. This activity is associated to TREK-1 or TREK-2 channels, because TRAAK channels are less sensitive to acidification than to alkalinization [14,15][14][15]. Furthermore, this channel showed a clear outward rectification at equimolar K+ concentrations, and the conductance of the individual channel measured during the outward current was twice that when measured during the inward current. With these data, we can speculate that the channels described in rat atrial cells corresponded to TREK-1-like channels. Finally, another TREK-like activity was identified when a stretch-activated K+ conductance was demonstrated in adult rat atrial cells [26], although a native TREK-1-like current was first characterized later in rat ventricular cardiomyocytes. In whole-cell experiments, an I TREK current was activated by adenosine triphosphate (ATP) via AA release after phospholipase A 2 (PLA 2) activation [74][72]. The activation of this current produces a clear hyperpolarization [93][91] that could be attributed to TREK channels [94][92]. Finally, the functional presence of TREK-1 has been revealed in KO animals, both in cardiomyocytes [95][93] and pacemaker cells of the SAN, where it plays an essential role in repolarization and in spontaneous nodal activity [88][86].
The TREK subfamily also seems to play a relevant role in several pathological heart conditions. For instance, TREK-1 overexpression is evident in rat ventricular cardiomyocytes in situations of isoproterenol-induced hypertrophy [86][84], whereas experimental deletion of TREK-1 impeded the recovery of an ex vivo-induced ischemia phase. In vivo, these animals showed a significantly longer QT interval and higher postinfarction mortality [95][93]. Similar data were also obtained in the heart of TREK-1 KO mice, with a significantly longer R-R (time elapsed between two successive R-waves of the QRS signal on the electrocardiogram) and QTc intervals (the interval between depolarization and the corrected ventricular repolarization), suggesting clear SAN dysfunction with the possibility of bradycardia or supraventricular arrhythmias [88][86]. Porcine models of AF have shown a clear downregulation of TREK-1 in atrial regions [70][68], and it was suggested that TREK-1 fulfils a clear role in arrhythmiogenesis [88][86]. In this sense, dronedarone is one of the drugs most often used in this pathology and as other known antiarrhythmic drugs such as vernakalant clearly inhibit TREK channels [96][94]. These data support the involvement of TREK in fibrillary processes, and they make these channels potential therapeutic targets for the treatment of AF and other heart diseases [56]. Finally, diltiazem (a calcium channel blocker with antiarrhythmic effect) inhibits both TREK-1 and TREK-2 [97][95], reinforcing the hypothesis that TREKs, and more specifically TREK-1, play an essential role in cardiac pathophysiology [89,98][87][96].
From a functional point of view, TREK-1 appears to counteract the depolarizing effect produced by stretch-activated cationic currents, contributing to stimulation-activated feedback mechanics in the heart [83,99,100][81][97][98]. It has been suggested that its experimental withdrawal could be proarrhythmic [89][87]. In addition, the uneven expression of TREK-1 in different cardiac regions would contribute to a tighter control of the depolarizing wave generated during cardiac contraction [2]. In this regard, it is known that in epicardial myocytes of adult rats there is less TREK-1 than in endocardial cells [85][83].
References
- Lesage, F.; Guillemare, E.; Fink, M.; Duprat, F.; Lazdunski, M.; Romey, G.; Barhanin, J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J. 1996, 15, 1004–1011.
- Enyedi, P.; Czirják, G. Molecular Background of Leak K+ Currents: Two-Pore Domain Potassium Channels. Physiol. Rev. 2010, 90, 559–605.
- Kang, D.; Kim, D. Single-channel properties and pH sensitivity of two-pore domain K+ channels of the TALK family. Biochem. Biophys. Res. Commun. 2004, 315, 836–844.
- Enyedi, P.; Czirjak, G. Properties, regulation, pharmacology, and functions of the K2P channel, TRESK. Pflügers Arch. Eur. J. Physiol. 2014, 467, 945–958.
- Piechotta, P.L.; Rapedius, M.; Stansfeld, P.J.; Bollepalli, M.K.; Erhlich, G.; Andres-Enguix, I.; Fritzenschaft, H.; Decher, N.; Sansom, M.S.P.; Tucker, S.J.; et al. The pore structure and gating mechanism of K2P channels. EMBO J. 2011, 30, 3607–3619.
- Feliciangeli, S.; Chatelain, F.C.; Bichet, D.; Lesage, F. The family of K2Pchannels: Salient structural and functional properties. J. Physiol. 2015, 593, 2587–2603.
- Talley, E.M.; Solórzano, G.; Lei, Q.; Kim, D.; Bayliss, D.A. CNS Distribution of Members of the Two-Pore-Domain (KCNK) Potassium Channel Family. J. Neurosci. 2001, 21, 7491–7505.
- Talley, E.M.; Sirois, J.E.; Lei, Q.; Bayliss, D.A. Two-Pore-Domain (Kcnk) Potassium Channels: Dynamic Roles in Neuronal Function. Neuroscientist 2003, 9, 46–56.
- Meuth, S.G.; Budde, T.; Kanyshkova, T.; Broicher, T.; Munsch, T.; Pape, H.-C. Contribution of TWIK-Related Acid-Sensitive K+Channel 1 (TASK1) and TASK3 Channels to the Control of Activity Modes in Thalamocortical Neurons. J. Neurosci. 2003, 23, 6460–6469.
- Gabriel, A.; Abdallah, M.; Yost, C.S.; Winegar, B.D.; Kindler, C.H. Localization of the tandem pore domain K+ channel KCNK5 (TASK-2) in the rat central nervous system. Mol. Brain Res. 2002, 98, 153–163.
- Rajan, S.; Wischmeyer, E.; Karschin, C.; Preisig-Müller, R.; Grzeschik, K.-H.; Daut, J.; Karschin, A.; Derst, C. THIK-1 and THIK-2, a Novel Subfamily of Tandem Pore Domain K+ Channels. J. Biol. Chem. 2001, 276, 7302–7311.
- Bushell, T.; Clarke, C.; Mathie, A.; Robertson, B. Pharmacological characterization of a non-inactivating outward current observed in mouse cerebellar Purkinje neurones. Br. J. Pharmacol. 2002, 135, 705–712.
- Bista, P.; Cerina, M.; Ehling, P.; Leist, M.; Pape, H.-C.; Meuth, S.G.; Budde, T. The role of two-pore-domain background K+ (K2P) channels in the thalamus. Pflügers Arch. Eur. J. Physiol. 2014, 467, 895–905.
- Kim, Y.; Bang, H.; Gnatenco, C.; Kim, D. Synergistic interaction and the role of C-terminus in the activation of TRAAK K+ channels by pressure, free fatty acids and alkali. Pflügers Arch. Eur. J. Physiol. 2001, 442, 64–72.
- Han, J.; Gnatenco, C.; Sladek, C.D.; Kim, D. Background and tandem-pore potassium channels in magnocellular neurosecretory cells of the rat supraoptic nucleus. J. Physiol. 2003, 546, 625–639.
- Medhurst, A.D.; Rennie, G.; Chapman, C.G.; Meadows, H.; Duckworth, M.D.; Kelsell, R.E.; Gloger, I.I.; Pangalos, M.N. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Mol. Brain Res. 2001, 86, 101–114.
- Marsh, B.; Acosta, C.; Djouhri, L.; Lawson, S.N. Leak K+ channel mRNAs in dorsal root ganglia: Relation to inflammation and spontaneous pain behaviour. Mol. Cell. Neurosci. 2012, 49, 375–386.
- Kang, D.; Kim, G.-T.; Kim, E.-J.; La, J.-H.; Lee, J.-S.; Lee, E.-S.; Park, J.-Y.; Hong, S.-G.; Han, J. Lamotrigine inhibits TRESK regulated by G-protein coupled receptor agonists. Biochem. Biophys. Res. Commun. 2008, 367, 609–615.
- Kang, D.; Kim, D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am. J. Physiol. Cell Physiol. 2006, 291, C138–C146.
- Lengyel, M.; Czirják, G.; Enyedi, P. Formation of Functional Heterodimers by TREK-1 and TREK-2 Two-pore Domain Potassium Channel Subunits. J. Biol. Chem. 2016, 291, 13649–13661.
- Cadaveira-Mosquera, A.; Pérez, M.; Reboreda, A.; Rivas-Ramírez, P.; Fernández-Fernández, D.; Lamas, J.A. Expression of K2P Channels in Sensory and Motor Neurons of the Autonomic Nervous System. J. Mol. Neurosci. 2012, 48, 86–96.
- Cadaveira-Mosquera, A.; Ribeiro, S.J.; Reboreda, A.; Perez, M.; Lamas, J.A. Activation of TREK Currents by the Neuroprotective Agent Riluzole in Mouse Sympathetic Neurons. J. Neurosci. 2011, 31, 1375–1385.
- Rivas-Ramírez, P.; Cadaveira-Mosquera, A.; Lamas, J.A.; Reboreda, A. Muscarinic modulation of TREK currents in mouse sympathetic superior cervical ganglion neurons. Eur. J. Neurosci. 2015, 42, 1797–1807.
- Fernández-Fernández, D.; Cadaveira-Mosquera, A.; Rueda-Ruzafa, L.; Herrera-Pérez, S.; Veale, E.L.; Reboreda, A.; Mathie, A.; Lamas, J.A. Activation of TREK currents by riluzole in three subgroups of cultured mouse nodose ganglion neurons. PLoS ONE 2018, 13, e0199282.
- Backx, P.H.; Marban, E. Background potassium current active during the plateau of the action potential in guinea pig ventricular myocytes. Circ. Res. 1993, 72, 890–900.
- Kim, D. A mechanosensitive K+ channel in heart cells. Activation by arachidonic acid. J. Gen. Physiol. 1992, 100, 1021–1040.
- Kim, D.; Clapham, D. Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science 1989, 244, 1174–1176.
- Liu, W.; Saint, D.A. Heterogeneous expression of tandem-pore K+ channel genes in adult and embryonic rat heart quantified by re-al-time polymerase chain reaction. Clin. Exp. Pharmacol. Physiol. 2004, 31, 174–178.
- Putzke, C.; Wemhöner, K.; Sachse, F.; Rinné, S.; Schlichthörl, G.; Li, X.T.; Jaé, L.; Eckhardt, I.; Wischmeyer, E.; Wulf, H.; et al. The acid-sensitive potassium channel TASK-1 in rat cardiac muscle. Cardiovasc. Res. 2007, 75, 59–68.
- Decher, N.; Wemhöner, K.; Rinné, S.; Netter, M.F.; Zuzarte, M.; Aller, M.I.; Kaufmann, S.G.; Li, X.T.; Meuth, S.G.; Daut, J.; et al. Knock-Out of the Potassium Channel TASK-1 Leads to a Prolonged QT Interval and a Disturbed QRS Complex. Cell. Physiol. Biochem. 2011, 28, 77–86.
- Meadows, H.J.; Chapman, C.G.; Duckworth, D.M.; Kelsell, R.E.; Murdock, P.R.; Nasir, S.; Rennie, G.; Randall, A.D. The neuroprotective agent sipatrigine (BW619C89) potently inhibits the human tandem pore-domain K+ channels TREK-1 and TRAAK. Brain Res. 2001, 892, 94–101.
- Gu, W.; Schlichthörl, G.; Hirsch, J.R.; Engels, H.; Karschin, C.; Karschin, A.; Derst, C.; Steinlein, O.K.; Daut, J. Expression pattern and functional characteristics of two novel splice variants of the two-pore-domain potassium channel TREK-2. J. Physiol. 2002, 539, 657–668.
- Terrenoire, C.; Lauritzen, I.; Lesage, F.; Romey, G.; Lazdunski, M. A TREK-1-like potassium channel in atrial cells inhibited by be-ta-adrenergic stimulation and activated by volatile anesthetics. Circ. Res. 2001, 89, 336–342.
- Schmidt, C.; Wiedmann, F.; Voigt, N.; Zhou, X.-B.; Heijman, J.; Lang, S.; Albert, V.; Kallenberger, S.; Ruhparwar, A.; Szabó, G.; et al. Upregulation of K 2P 3.1 K + Current Causes Action Potential Shortening in Patients with Chronic Atrial Fibrillation. Circulation 2015, 132, 82–92.
- Chavez, R.A.; Gray, A.T.; Zhao, B.B.; Kindler, C.H.; Mazurek, M.J.; Mehta, Y.; Forsayeth, J.R.; Yost, C.S. TWIK-2, a New Weak Inward Rectifying Member of the Tandem Pore Domain Potassium Channel Family. J. Biol. Chem. 1999, 274, 7887–7892.
- Thomas, D.; Goldstein, S.A.N. Two-P-Domain (K2P) Potassium Channels: Leak Conductance Regulators of Excitability. In EnCyclopedia of Neuroscience; Academic Press: Cambridge, MA, USA, 2009; pp. 1207–1220.
- Patel, A.J.; Maingret, F.; Magnone, V.; Fosset, M.; Lazdunski, M.; Honoré, E. TWIK-2, an Inactivating 2P Domain K+ Channel. J. Biol. Chem. 2000, 275, 28722–28730.
- Wang, Z.; Yue, L.; White, M.; Pelletier, G.; Nattel, S. Differential Distribution of Inward Rectifier Potassium Channel Transcripts in Human Atrium Versus Ventricle. Circulation 1998, 98, 2422–2428.
- Ellinghaus, P.; Scheubel, R.J.; Dobrev, D.; Ravens, U.; Holtz, J.; Huetter, J.; Nielsch, U.; Morawietz, H. Comparing the global mRNA expression profile of human atrial and ventricular myocardium with high-density oligonucleotide arrays. J. Thorac. Cardiovasc. Surg. 2005, 129, 1383–1390.
- Gaborit, N.; Steenman, M.; Lamirault, G.; Le Meur, N.; Le Bouter, S.; Lande, G.; Léger, J.; Charpentier, F.; Christ, T.; Dobrev, D.; et al. Human Atrial Ion Channel and Transporter Subunit Gene-Expression Remodeling Associated with Valvular Heart Disease and Atrial Fibrillation. Circulation 2005, 112, 471–481.
- Christensen, A.H.; Chatelain, F.C.; Huttner, I.G.; Olesen, M.S.; Soka, M.; Feliciangeli, S.; Horvat, C.; Santiago, C.F.; Vandenberg, J.I.; Schmitt, N.; et al. The two-pore domain potassium channel, TWIK-1, has a role in the regulation of heart rate and atrial size. J. Mol. Cell. Cardiol. 2016, 97, 24–35.
- Gaborit, N.; Le Bouter, S.; Szüts, V.; Varro, A.; Escande, D.; Nattel, S.; Demolombe, S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J. Physiol. 2007, 582, 675–693.
- Kim, N.; Fujita, A.; Horio, Y.; Kurachi, Y. Cloning and Functional Expression of a Novel Cardiac Two-Pore Background K + Channel (cTBAK-1). Circ. Res. 1998, 82, 513–518.
- Donner, B.C.; Schullenberg, M.; Geduldig, N.; Hüning, A.; Mersmann, J.; Zacharowski, K.; Kovacevic, A.; Decking, U.; Aller, M.I.; Schmidt, K.G. Functional role of TASK-1 in the heart: Studies in TASK-1-deficient mice show prolonged cardiac repolarization and reduced heart rate variability. Basic Res. Cardiol. 2010, 106, 75–87.
- Amin, A.S.; Wilde, A.A.M. Inheritable Potassium Channel Diseases. In Cardiac Electrophysiology: From Cell to Bedside, 7th ed.; Zipes, D.P., Jalife, J., Stevenson, W.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 494–503.
- Bartos, D.C.; Grandi, E.; Ripplinger, C.M. Ion Channels in the Heart. Compr. Physiol. 2015, 5, 1423–1464.
- Oudit, G.Y.; Backx, P.H. Voltage-Gated Potassium Channels. In Cardiac Electrophysiology: From Cell to Bedside, 7th ed.; Zipes, D.P., Jalife, J., Stevenson, W.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 25–37.
- Mackaay, A.J.; Op’t Hof, T.; Bleeker, W.K.; Jongsma, H.J.; Bouman, L.N. Interaction of adrenaline and acetylcholine on cardiac pace-maker function. Functional inhomogeneity of the rabbit sinus node. J. Pharmacol. Exp. Ther. 1980, 214, 417–422.
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666.
- Chen, S.-A.; Hsieh, M.-H.; Tai, C.-T.; Tsai, C.-F.; Prakash, V.S.; Yu, W.-C.; Hsu, T.-L.; Ding, Y.-A.; Chang, M.-S. Initiation of Atrial Fibrillation by Ectopic Beats Originating from the Pulmonary Veins. Circulation 1999, 100, 1879–1886.
- Nishida, K.; Maguy, A.; Sakabe, M.; Comtois, P.; Inoue, H.; Nattel, S. The role of pulmonary veins vs. autonomic ganglia in different experimental substrates of canine atrial fibrillation. Cardiovasc. Res. 2010, 89, 825–833.
- Filgueiras-Rama, D. Sympathetic Innervation and Cardiac Arrhythmias. In Cardiac Electrophysiology: From Cell to Bedside, 7th ed.; Douglas, P., Zipes, J.J., William, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 387–395.
- Brack, K.E.; Winter, J.; Ng, G.A. Mechanisms underlying the autonomic modulation of ventricular fibrillation initiation—tentative prophylactic properties of vagus nerve stimulation on malignant arrhythmias in heart failure. Hear. Fail. Rev. 2012, 18, 389–408.
- Ma, L.; Zhang, X.; Chen, H. TWIK-1 Two-Pore Domain Potassium Channels Change Ion Selectivity and Conduct Inward Leak Sodium Currents in Hypokalemia. Sci. Signal. 2011, 4, ra37.
- Pezhouman, A.; Singh, N.; Song, Z.; Nivala, M.; Eskandari, A.; Cao, H.; Bapat, A.; Ko, C.Y.; Nguyen, T.; Qu, Z.; et al. Molecular Basis of Hypokalemia-Induced Ventricular Fibrillation. Circulation 2015, 132, 1528–1537.
- Schmidt, C.; Wiedmann, F.; Schweizer, P.A.; Becker, R.; Katus, H.A.; Thomas, D. Novel electrophysiological properties of dronedarone: Inhibition of human cardiac two-pore-domain potassium (K2P) channels. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 1003–1016.
- Gaborit, N.; Wichter, T.; Varro, A.; Szuts, V.; Lamirault, G.; Eckardt, L.; Paul, M.; Breithardt, G.; Schulze-Bahr, E.; Escande, D.; et al. Transcrip-tional profiling of ion channel genes in Brugada syndrome and other right ventricular arrhythmogenic diseases. Eur. Heart J. 2009, 30, 487–496.
- Duprat, F.; Lesage, F.; Fink, M.; Reyes, R.; Heurteaux, C.; Lazdunski, M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997, 16, 5464–5471.
- Kim, D.; Gnatenco, C. TASK-5, a New Member of the Tandem-Pore K+ Channel Family. Biochem. Biophys. Res. Commun. 2001, 284, 923–930.
- Meuth, S.G.; Kleinschnitz, C.; Broicher, T.; Austinat, M.; Braeuninger, S.; Bittner, S.; Fischer, S.; Bayliss, D.A.; Budde, T.; Stoll, G.; et al. The neuroprotective impact of the leak potassium channel TASK1 on stroke development in mice. Neurobiol. Dis. 2009, 33, 1–11.
- Lopes, C.M.; Gallagher, P.G.; Buck, M.E.; Butler, M.H.; Goldstein, S.A. Proton Block and Voltage Gating Are Potassium-dependent in the Cardiac Leak Channel Kcnk3. J. Biol. Chem. 2000, 275, 16969–16978.
- Kim, Y.; Bang, H.; Kim, D. TBAK-1 and TASK-1, two-pore K(+) channel subunits: Kinetic properties and expression in rat heart. Am. J. Physiol. Heart Circ. Physiol. 1999, 277, H1669–H1678.
- Leonoudakis, D.; Gray, A.T.; Winegar, B.D.; Kindler, C.H.; Harada, M.; Taylor, D.M.; Chavez, R.A.; Forsayeth, J.R.; Yost, C.S. An Open Rectifier Potassium Channel with Two Pore Domains in Tandem Cloned from Rat Cerebellum. J. Neurosci. 1998, 18, 868–877.
- Friedrich, C.; Rinné, S.; Zumhagen, S.; Kiper, A.K.; Silbernagel, N.; Netter, M.F.; Stallmeyer, B.; Schulze-Bahr, E.; Decher, N. Gain-of-function mutation in TASK-4 channels and severe cardiac conduction disorder. EMBO Mol. Med. 2014, 6, 937–951.
- Limberg, S.H.; Netter, M.F.; Rolfes, C.; Rinné, S.; Schlichthörl, G.; Zuzarte, M.; Vassiliou, T.; Moosdorf, R.; Wulf, H.; Daut, J.; et al. TASK-1 Channels May Modulate Action Potential Duration of Human Atrial Cardiomyocytes. Cell. Physiol. Biochem. 2011, 28, 613–624.
- Graham, V.; Zhang, H.; Willis, S.; Creazzo, T.L. Expression of a two-pore domain K+ channel (TASK-1) in developing avian and mouse ventricular conduction systems. Dev. Dyn. 2005, 235, 143–151.
- Decher, N.; Kiper, A.K.; Rolfes, C.; Schulze-Bahr, E.; Rinne, S. The role of acid-sensitive two-pore domain potassium channels in cardiac electrophysiology: Focus on arrhythmias. Pflugers Arch 2015, 467, 1055–1067.
- Schmidt, C.; Wiedmann, F.; Tristram, F.; Anand, P.; Wenzel, W.; Lugenbiel, P.; Schweizer, P.A.; Katus, H.A.; Thomas, D. Cardiac expression and atrial fibrillation-associated remodeling of K2P2.1 (TREK-1) K+ channels in a porcine model. Life Sci. 2014, 97, 107–115.
- Schmidt, C.; Wiedmann, F.; Beyersdorf, C.; Zhao, Z.; El-Battrawy, I.; Lan, H.; Szabo, G.; Li, X.; Lang, S.; Korkmaz-Icöz, S.; et al. Genetic Ablation of TASK-1 (Tandem of P Domains in a Weak Inward Rectifying K + Channel–Related Acid-Sensitive K + Channel-1) (K 2P 3.1) K + Channels Suppresses Atrial Fibrillation and Prevents Electrical Remodeling. Circ. Arrhythmia Electrophysiol. 2019, 12, e007465.
- Williams, S.; Bateman, A.; O’Kelly, I. Altered Expression of Two-Pore Domain Potassium (K2P) Channels in Cancer. PLoS ONE 2013, 8, e74589.
- Maingret, F.; Fosset, M.; Lesage, F.; Lazdunski, M.; Honoré, E. TRAAK Is a Mammalian Neuronal Mechano-gated K+Channel. J. Biol. Chem. 1999, 274, 1381–1387.
- Aimond, F.; Rauzier, J.-M.; Bony, C.; Vassort, G. Simultaneous Activation of p38 MAPK and p42/44 MAPK by ATP Stimulates the K+ Current ITREK in Cardiomyocytes. J. Biol. Chem. 2000, 275, 39110–39116.
- Lesage, F.; Maingret, F.; Lazdunski, M. Cloning and expression of human TRAAK, a polyunsaturated fatty acids-activated and mecha-no-sensitive K+ channel. FEBS Lett. 2000, 471, 137–140.
- Chemin, J.; Patel, A.J.; Duprat, F.; Lauritzen, I.; Lazdunski, M.; Honoré, E. A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J. 2005, 24, 44–53.
- Patel, A.J.; Honoré, E.; Maingret, F.; Lesage, F.; Fink, M.; Duprat, F.; Lazdunski, M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998, 17, 4283–4290.
- Lamas, J.A. Mechanosensitive K2P channels, TREKking through the autonomic nervous system. In Mechanically Gated Channels and Their Regulation; Kamkin, A., Lozinsky, I., Eds.; Mechanosensitivity in Cells and Tissues; Springer Science + Business Media: Dordrecht, The Netherlands, 2012; Volume 6, pp. 35–68.
- Mathie, A.; Veale, E.L. Two-pore domain potassium channels: Potential therapeutic targets for the treatment of pain. Pflügers Arch. Eur. J. Physiol. 2015, 467, 931–943.
- Brohawn, S.G.; Campbell, E.B.; MacKinnon, R. Physical mechanism for gating and mechanosensitivity of the human TRAAK K+ channel. Nature 2014, 516, 126–130.
- Noël, J.; Zimmermann, K.; Busserolles, J.; Deval, E.; Alloui, A.; Diochot, S.; Guy, N.; Borsotto, M.; Reeh, P.; Eschalier, A.; et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009, 28, 1308–1318.
- Lamas, J.A.; Rueda-Ruzafa, L.; Herrera-Pérez, S. Ion Channels and Thermosensitivity: TRP, TREK, or Both? Int. J. Mol. Sci. 2019, 20, 2371.
- Li, X.T.; Dyachenko, V.; Zuzarte, M.; Putzke, C.; Preisig-Müller, R.; Isenberg, G.; Daut, J. The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovasc. Res. 2006, 69, 86–97.
- Fink, M.; Duprat, F.; Lesage, F.; Reyes, R.; Romey, G.; Heurteaux, C.; Lazdunski, M. Cloning, functional expression and brain localiza-tion of a novel unconventional outward rectifier K+ channel. EMBO J. 1996, 15, 6854–6862.
- Tan, J.H.C.; Liu, W.; Saint, D.A. Differential expression of the mechanosensitive potassium channelTREK-1in epicardial and endocardial myocytes in rat ventricle. Exp. Physiol. 2004, 89, 237–242.
- Wang, W.; Zhang, M.; Li, P.; Yuan, H.; Feng, N.; Peng, Y.; Wang, L.; Wang, X. An Increased TREK-1–like Potassium Current in Ventricular Myocytes During Rat Cardiac Hypertrophy. J. Cardiovasc. Pharmacol. 2013, 61, 302–310.
- Abraham, D.M.; Lee, T.E.; Watson, L.J.; Mao, L.; Chandok, G.; Wang, H.G.; Frangakis, S.; Pitt, G.S.; Shah, S.H.; Wolf, M.J.; et al. The two-pore domain potassium channel TREK-1 mediates cardiac fibrosis and diastolic dysfunction. J. Clin. Investig. 2018, 128, 4843–4855.
- Unudurthi, S.D.; Wu, X.; Qian, L.; Amari, F.; Onal, B.; Li, N.; Makara, M.A.; Smith, S.A.; Snyder, J.; Fedorov, V.V.; et al. Two-Pore K + Channel TREK-1 Regulates Sinoatrial Node Membrane Excitability. J. Am. Heart Assoc. 2016, 5.
- Decher, N.; Kiper, A.K.; Rinné, S. Stretch-activated potassium currents in the heart: Focus on TREK-1 and arrhythmias. Prog. Biophys. Mol. Biol. 2017, 130, 223–232.
- Wiedmann, F.; Rinné, S.; Donner, B.; Decher, N.; Katus, H.A.; Schmidt, C. Mechanosensitive TREK-1 two-pore-domain potassium (K2P) channels in the cardiovascular system. Prog. Biophys. Mol. Biol. 2021, 159, 126–135.
- Fink, M.; Lesage, F.; Duprat, F.; Heurteaux, C.; Reyes, R.; Fosset, M.; Lazdunski, M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 1998, 17, 3297–3308.
- Ozaita, A.; de Miera, E.V.S. Cloning of two transcripts, HKT4.1a and HKT4.1b, from the human two-pore K+ channel gene KCNK4: Chromosomal localization, tissue distribution and functional expression. Mol. Brain Res. 2002, 102, 18–27.
- Kohl, P.; Bollensdorff, C.; Garny, A. Effects of mechanosensitive ion channels on ventricular electrophysiology: Experimental and theoretical models. Exp. Physiol. 2006, 91, 307–321.
- Schmidt, C.; Peyronnet, R. Voltage-gated and stretch-activated potassium channels in the human heart. Herzschrittmachertherapie Elektrophysiologie 2018, 29, 36–42.
- Kamatham, S.; Waters, C.M.; Schwingshackl, A.; Mancarella, S. TREK-1 protects the heart against ischemia-reperfusion-induced injury and from adverse remodeling after myocardial infarction. Pflügers Arch. Eur. J. Physiol. 2019, 471, 1263–1272.
- Seyler, C.; Li, J.; Schweizer, P.A.; Katus, H.A.; Thomas, D. Inhibition of cardiac two-pore-domain K+ (K2P) channels by the antiarrhythmic drug vernakalant—Comparison with flecainide. Eur. J. Pharmacol. 2014, 724, 51–57.
- Takahira, M.; Sakurai, M.; Sakurada, N.; Sugiyama, K. Fenamates and diltiazem modulate lipid-sensitive mechano-gated 2P domain K+ channels. Pflügers Arch. Eur. J. Physiol. 2005, 451, 474–478.
- Decher, N.; Ortiz-Bonnin, B.; Friedrich, C.; Schewe, M.; Kiper, A.K.; Rinné, S.; Seemann, G.; Peyronnet, R.; Zumhagen, S.; Bustos, D.; et al. Sodium permeable and “hypersensitive” TREK-1 channels cause ventricular tachycardia. EMBO Mol. Med. 2017, 9, 403–414.
- Goonetilleke, L.; Quayle, J. TREK-1 K+ Channels in the Cardiovascular System: Their Significance and Potential as a Therapeutic Target. Cardiovasc. Ther. 2012, 30, e23–e29.
- Kohl, P. Cardiac Stretch-Activated Channels and Mechano-Electric Coupling. In Cardiac Electrophysiology: From Cell to Bedside, 7th ed.; Douglas, P., Zipes, J.J., William, G., Stevenson, Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 128–139.
