Cancer cachexia is a syndrome experienced by many patients with cancer. Exercise can act as an autophagy modulator, and thus holds the potential to be used to treat cancer cachexia. Autophagy imbalance plays an important role in cancer cachexia, and is correlated to skeletal and cardiac muscle atrophy and energy-wasting in the liver. The molecular mechanism of autophagy modulation in different types of exercise has not yet been clearly defined. This review aims to elaborate on the role of exercise in modulating autophagy in cancer cachexia.
- cancer
- cachexia
- autophagy
- combined exercise
- aerobic
- resistance
1. Overview
Cancer cachexia is a syndrome experienced by many patients with cancer. Exercise can act as an autophagy modulator, and thus holds the potential to be used to treat cancer cachexia. Autophagy imbalance plays an important role in cancer cachexia, and is correlated to skeletal and cardiac muscle atrophy and energy-wasting in the liver. The molecular mechanism of autophagy modulation in different types of exercise has not yet been clearly defined. This review aims to elaborate on the role of exercise in modulating autophagy in cancer cachexia. We evaluated nine studies in the literature and found a potential correlation between the type of exercise and autophagy modulation. Combined exercise or aerobic exercise alone seems more beneficial than resistance exercise alone in cancer cachexia. Looking ahead, determining the physiological role of autophagy modulated by exercise will support the development of a new medical approach for treating cancer cachexia. In addition, the harmonization of the exercise type, intensity, and duration might play a key role in optimizing the autophagy levels to preserve muscle function and regulate energy utilization in the liver.
2. Cachexia
3. Changes of Autophagy in Organs Related to Cancer Cachexia
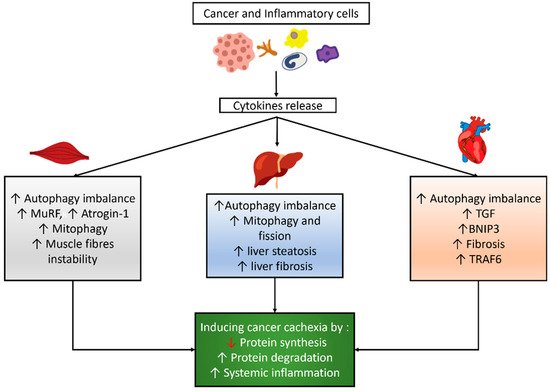
3.1. Autophagy Modulation in the Skeletal Muscle of Cancer Cachexia
3.2. Autophagy Modulation in the Cardiac Muscle of Cancer Cachexia
3.3. Autophagy Modulation in the Liver of Cancer Cachexia
References
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495.
- Yang, W.; Huang, J.; Wu, H.; Wang, Y.; Du, Z.; Ling, Y.; Wang, W.; Wu, Q.; Gao, W. Molecular mechanisms of cancer cachexia-induced muscle atrophy (Review). Mol. Med. Rep. 2020, 22, 4967–4980.
- Sadeghi, M.A.; Keshavarz-Fathi, M.; Baracos, V.; Arends, J.; Mahmoudi, M.; Rezaei, N. Cancer cachexia: Diagnosis, assessment, and treatment. Crit. Rev. Oncol. 2018, 127, 91–104.
- Tazi, E.; Errihani, H. Treatment of cachexia in oncology. Indian J. Palliat. Care 2010, 16, 136–137.
- de Castro Gonçalves, R.; Freire, P.P.; Coletti, D.; Seelaender, M. Tumor Microenvironment Autophagic Processes and Cachexia: The Missing Link? Front. Oncol. 2021, 10, 3170.
- Da Silva, S.P.; Santos, J.M.O.; Silva, M.P.C.E.; da Costa, R.M.G.; Medeiros, R. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. J. Cachexia Sarcopenia Muscle 2020, 11, 619–635.
- Siddiqui, J.A.; Pothuraju, R.; Jain, M.; Batra, S.K.; Nasser, M.W. Advances in cancer cachexia: Intersection between affected organs, mediators, and pharmacological interventions. Biochim. Biophys. Acta (BBA) Rev. Cancer 2020, 1873, 188359.
- Chen, H.-Y.; White, E. Role of Autophagy in Cancer Prevention. Cancer Prev. Res. 2011, 4, 973–983.
- Chavez-Dominguez, R.; Perez-Medina, M.; Lopez-Gonzalez, J.S.; Galicia-Velasco, M.; Aguilar-Cazares, D. The Double-Edge Sword of Autophagy in Cancer: From Tumor Suppression to Pro-tumor Activity. Front. Oncol. 2020, 10, 578418.
- Levy, J.M.M.; Thorburn, A. Autophagy in cancer: Moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 2020, 27, 843–857.
- Leal, L.G.; Lopes, M.A.; Peres, S.B.; Batista, M.L.J. Exercise Training as Therapeutic Approach in Cancer Cachexia: A Review of Potential Anti-inflammatory Effect on Muscle Wasting. Front. Physiol. 2021, 11, 1769.
- American College of Sports Medicine. Exercise Testing and Prescription for Populations with Other Chronic Diseases and Health Conditions. In ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Riebe, D., Ehrman, J.K., Liguori, G., Magal, M., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2018; pp. 302–311.
- Niels, T.; Tomanek, A.; Freitag, N.; Schumann, M. Can Exercise Counteract Cancer Cachexia? A Systematic Literature Review and Meta-Analysis. Integr. Cancer Ther. 2020, 19.
- Solheim, T.S.; Laird, B.J.; Balstad, T.R.; Stene, G.B.; Bye, A.; Johns, N.; Pettersen, C.H.; Fallon, M.; Fayers, P.; Fearon, K.; et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 778–788.
- Hardee, J.P.; Counts, B.R.; Carson, J.A. Understanding the Role of Exercise in Cancer Cachexia Therapy. Am. J. Lifestyle Med. 2017, 13, 46–60.
- Pigna, E.; Berardi, E.; Aulino, P.; Rizzuto, E.; Zampieri, S.; Carraro, U.; Kern, H.; Merigliano, S.; Gruppo, M.; Mericskay, M.; et al. Aerobic Exercise and Pharmacological Treatments Counteract Cachexia by Modulating Autophagy in Colon Cancer. Sci. Rep. 2016, 6, 26991.
- Ballarò, R.; Penna, F.; Pin, F.; Gómez-Cabrera, M.C.; Viña, J.; Costelli, P. Moderate Exercise Improves Experimental Cancer Cachexia by Modulating the Redox Homeostasis. Cancers 2019, 11, 285.
- Ranjbar, K.; Ballarò, R.; Bover, Q.; Pin, F.; Beltrà, M.; Penna, F.; Costelli, P. Combined Exercise Training Positively Affects Muscle Wasting in Tumor-Bearing Mice. Med. Sci. Sports Exerc. 2019, 51, 1387–1395.
- Fernandes, L.G.; Tobias, G.C.; Paixão, A.O.; Dourado, P.M.; Voltarelli, V.A.; Brum, P.C. Exercise training delays cardiac remodeling in a mouse model of cancer cachexia. Life Sci. 2020, 260, 118392.
- Parry, T.L.; Hayward, R. Exercise Protects against Cancer-induced Cardiac Cachexia. Med. Sci. Sports Exerc. 2018, 50, 1169–1176.
- Morinaga, M.; Sako, N.; Isobe, M.; Lee-Hotta, S.; Sugiura, H.; Kametaka, S. Aerobic Exercise Ameliorates Cancer Cachexia-Induced Muscle Wasting through Adiponectin Signaling. Int. J. Mol. Sci. 2021, 22, 3110.
- Møller, A.B.; Lønbro, S.; Farup, J.; Voss, T.S.; Rittig, N.; Wang, J.; Højris, I.; Mikkelsen, U.R.; Jessen, N. Molecular and cellular adaptations to exercise training in skeletal muscle from cancer patients treated with chemotherapy. J. Cancer Res. Clin. Oncol. 2019, 145, 1449–1460.
- das Neves, W.; Alves, C.; de Almeida, N.R.; Guimarães, F.L.R.; Ramires, P.R.; Brum, P.C.; Lancha, A.H., Jr. Loss of strength capacity is associated with mortality, but resistance exercise training promotes only modest effects during cachexia progression. Life Sci. 2016, 163, 11–22.
- Ballarò, R.; Lopalco, P.; Audrito, V.; Beltrà, M.; Pin, F.; Angelini, R.; Costelli, P.; Corcelli, A.; Bonetto, A.; Szeto, H.; et al. Targeting Mitochondria by SS-31 Ameliorates the Whole Body Energy Status in Cancer- and Chemotherapy-Induced Cachexia. Cancers 2021, 13, 850.
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873.
- Das, G.; Shravage, B.V.; Baehrecke, E.H. Regulation and Function of Autophagy during Cell Survival and Cell Death. Cold Spring Harb. Perspect. Biol. 2012, 4, a008813.
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466.
- Mizushima, N.; Yamamoto, A.; Matsui, M.; Yoshimori, T.; Ohsumi, Y. In Vivo Analysis of Autophagy in Response to Nutrient Starvation Using Transgenic Mice Expressing a Fluorescent Autophagosome Marker. Mol. Biol. Cell 2004, 15, 1101–1111.
- Grumati, P.; Coletto, L.; Schiavinato, A.; Castagnaro, S.; Bertaggia, E.; Sandri, M.; Bonaldo, P. Physical exercise stimulates autophagy in normal skeletal muscles but is detrimental for collagen VI-deficient muscles. Autophagy 2011, 7, 1415–1423.
- Carnio, S.; LoVerso, F.; Baraibar, M.A.; Longa, E.; Khan, M.M.; Maffei, M.; Reischl, M.; Canepari, M.; Löfler, S.; Kern, H.; et al. Autophagy Impairment in Muscle Induces Neuromuscular Junction Degeneration and Precocious Aging. Cell Rep. 2014, 8, 1509–1521.
- Brocca, L.; Cannavino, J.; Coletto, L.; Biolo, G.; Sandri, M.; Bottinelli, R.; Pellegrino, M.A. The time course of the adaptations of human muscle proteome to bed rest and the underlying mechanisms. J. Physiol. 2012, 590, 5211–5230.
- Penna, F.; Costamagna, D.; Pin, F.; Camperi, A.; Fanzani, A.; Chiarpotto, E.M.; Cavallini, G.; Bonelli, G.; Baccino, F.M.; Costelli, P. Autophagic Degradation Contributes to Muscle Wasting in Cancer Cachexia. Am. J. Pathol. 2013, 182, 1367–1378.
- Sukari, A.; Muqbil, I.; Mohammad, R.M.; Philip, P.A.; Azmi, A.S. F-BOX proteins in cancer cachexia and muscle wasting: Emerging regulators and therapeutic opportunities. Semin. Cancer Biol. 2016, 36, 95–104.
- Rausch, V.; Sala, V.; Penna, F.; Porporato, P.E.; Ghigo, A. Understanding the common mechanisms of heart and skeletal muscle wasting in cancer cachexia. Oncogenesis 2021, 10, 1–13.
- Hentilä, J.; Nissinen, T.A.; Korkmaz, A.; Lensu, S.; Silvennoinen, M.; Pasternack, A.; Ritvos, O.; Atalay, M.; Hulmi, J.J. Activin Receptor Ligand Blocking and Cancer Have Distinct Effects on Protein and Redox Homeostasis in Skeletal Muscle and Liver. Front. Physiol. 2019, 9, 9.
- Zheng, Y.; Chen, H.; Li, X.; Sun, Y. Pay attention to cardiac remodeling in cancer cachexia. Support. Care Cancer 2016, 24, 3253–3259.
- Belloum, Y.; Rannou-Bekono, F.; Favier, F.B. Cancer-induced cardiac cachexia: Pathogenesis and impact of physical activity. Oncol. Rep. 2017, 37, 2543–2552.
- Shum, A.M.Y.; Fung, D.C.Y.; Corley, S.M.; McGill, M.C.; Bentley, N.L.; Tan, T.C.; Wilkins, M.R.; Polly, P. Cardiac and skeletal muscles show molecularly distinct responses to cancer cachexia. Physiol. Genom. 2015, 47, 588–599.
- Cosper, P.; Leinwand, L.A. Cancer Causes Cardiac Atrophy and Autophagy in a Sexually Dimorphic Manner. Cancer Res. 2010, 71, 1710–1720.
- Belury, M.; Tian, M.; Asp, M.L.; Nishijima, Y. Evidence for cardiac atrophic remodeling in cancer-induced cachexia in mice. Int. J. Oncol. 2011, 39, 1321–1326.
- Hinch, E.C.A.; Sullivan-Gunn, M.J.; Vaughan, V.C.; McGlynn, M.A.; Lewandowski, P.A. Disruption of pro-oxidant and antioxidant systems with elevated expression of the ubiquitin proteosome system in the cachectic heart muscle of nude mice. J. Cachexia Sarcopenia Muscle 2013, 4, 287–293.
- Nishida, K.; Otsu, K. Autophagy during cardiac remodeling. J. Mol. Cell. Cardiol. 2016, 95, 11–18.
- Manne, N.D.; Lima, M.; Enos, R.T.; Wehner, P.; Carson, J.A.; Blough, E. Altered cardiac muscle mTOR regulation during the progression of cancer cachexia in the ApcMin/+ mouse. Int. J. Oncol. 2013, 42, 2134–2140.
- Rohm, M.; Zeigerer, A.; Machado, J.; Herzig, S. Energy metabolism in cachexia. EMBO Rep. 2019, 20, 47258.
- Grasmann, G.; Smolle, E.; Olschewski, H.; Leithner, K. Gluconeogenesis in cancer cells – Repurposing of a starvation-induced metabolic pathway? Biochim. Biophys. Acta Mol. Cell Res. 2019, 1872, 24–36.
- Warburg, O. The Metabolism of Carcinoma Cells. J. Cancer Res. 1925, 9, 148–163.
- Porporato, P.E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200.
- Gonçalves, D.C.; Lira, F.S.; Yamashita, A.S.; Junior, L.C.C.; Eder, R.; Laviano, A.; Seelaender, M.C.L. Liver lipid metabolism disruption in cancer cachexia is aggravated by cla supplementation -induced inflammation. Clin. Nutr. 2019, 38, 2219–2230.
- Peyta, L.; Jarnouen, K.; Pinault, M.; Coulouarn, C.; Guimaraes, C.; Goupille, C.; de Barros, J.-P.P.; Chevalier, S.; Dumas, J.-F.; Maillot, F.; et al. Regulation of hepatic cardiolipin metabolism by TNFα: Implication in cancer cachexia. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 1490–1500.
- Julienne, C.M.; Tardieu, M.; Chevalier, S.; Pinault, M.; Bougnoux, P.; Labarthe, F.; Couet, C.; Servais, S.; Dumas, J.-F. Cardiolipin content is involved in liver mitochondrial energy wasting associated with cancer-induced cachexia without the involvement of adenine nucleotide translocase. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 726–733.
- Rosa-Caldwell, M.E.; Brown, J.L.; Lee, D.E.; Wiggs, M.P.; Perry, R.A., Jr.; Haynie, W.S.; Caldwell, A.R.; Washington, T.A.; Lo, W.-J.; Greene, N.P. Hepatic alterations during the development and progression of cancer cachexia. Appl. Physiol. Nutr. Metab. 2020, 45, 500–512.
- Ke, P.-Y. Diverse Functions of Autophagy in Liver Physiology and Liver Diseases. Int. J. Mol. Sci. 2019, 20, 300.
- Baena, M.; Sangüesa, G.; Hutter, N.; Sánchez, R.M.; Roglans, N.; Laguna, J.C.; Alegret, M. Fructose supplementation impairs rat liver autophagy through mTORC activation without inducing endoplasmic reticulum stress. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 107–116.
- González-Rodríguez, Á.; Mayoral, R.; Agra, N.; Valdecantos, M.P.; Pardo, V.; Miquilena-Colina, M.E.; Vargas-Castrillón, J.; Iacono, O.L.; Corazzari, M.; Fimia, G.M.; et al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 2014, 5, e1179.
- Jiang, P.; Huang, Z.; Zhao, H.; Wei, T. Hydrogen peroxide impairs autophagic flux in a cell model of nonalcoholic fatty liver disease. Biochem. Biophys. Res. Commun. 2013, 433, 408–414.
