Ciguatera poisoning is regarded globally as the most significant non-bacterial poisoning associated with fish consumption. It is usually limited to the consumption of toxic fish from regions between the latitudes 35° N and 35° S. Studies have shown a strong positive correlation between Gambierdiscus abundance and algal macrophytes. Some earlier studies proposed a standardized methodology for estimating Gambierdiscus abundance based on sampling macroalgae.
- the Indian Ocean
- Arabian sea
- Kuwait bay
- Aden Gulf
- Red Sea
- Gulf of Aqaba
- Andaman Sea
- Bay of Bengal
- seafood safety
- foodborne disease
1. Introduction
Ciguatera poisoning (CP) is a syndrome caused by ingestion of coral reef fish and shellfish of tropical and subtropical regions, which has caused global concern. Some dinoflagellate species of the genera Gambierdiscus and Fukuyoa are known to produce ciguatoxins (CTXs); the organisms that consume these toxic algae accumulate CTXs that are transferred and biotransformed along the marine and human food chain. These lipid-soluble and heat-resistant toxins cause gastrointestinal, cardiovascular, and neurological disorders among humans consuming the CTX-contaminated seafood [1,2,3,4,5][1][2][3][4][5]. According to a study published in 2008, CP disease estimations are uncertain and often misdiagnosed [6]. However, few reports predict 2–10 million people are annually affected by CP [7,8][7][8].
Ciguatera poisoning is regarded globally as the most significant non-bacterial poisoning associated with fish consumption. It is usually limited to the consumption of toxic fish from regions between the latitudes 35° N and 35° S [9,10,11,12,13,14,15,16,17][9][10][11][12][13][14][15][16][17]. Studies have shown a strong positive correlation between Gambierdiscus abundance and algal macrophytes [18]. Some earlier studies proposed a standardized methodology for estimating Gambierdiscus abundance based on sampling macroalgae [19,20,21][19][20][21]. More recent studies brought to light the significant biases in Gambierdiscus cell distribution within the macrophytes. A 33–150% variation was reported among replicates [22,23,24][22][23][24]. Despite these limitations, some first-order estimates on Gambierdiscus distributions for large geographic regions have been attempted using the average abundances [11,25][11][25]. These have shown a global distribution spreading across oceans with 85% of Gambierdiscus density estimates <1000 cells g −1 wet weight algae, with <10% occurrence with 10–10,000 cells g −1 wet weight algae and <5% incidences where the concentration was >100,000 cells g −1 wet weight algae. With the growing global demands for seafood and international trade ease, CP concerns the population beyond the endemic regions and is increasingly becoming a global issue. Moreover, a greater threat is posed as there are no current antidotes for CP [26].
Historically, poisoning associated with seafood consumption was reported in different parts of the globe. It was first recounted in the West Indies as early as 1511 [27] and in the Gulf of Guinea in 1521, killing the Captains of the Spanish army [28]. It was also reported in the islands of the Indian Ocean in 1601 and various archipelagos of the Pacific Ocean in 1606 [27]. Additionally, in 1786, the surgeon of HMS Endeavour, en route to Australia, New Zealand, and the Pacific Islands, reported that the ship’s Captain was poisoned by ciguatoxins [29]. A year later, the ingestion of a local gastropod ( Livonia sp.) was discovered to induce neurological symptoms in the Antilles of the Caribbean Sea [30]. In 1948, the organism Gambierdiscus (originally referred to as Goniodoma sp.) was described for the first time in Cabo Verde [31]. The term “ciguatera poisoning” was coined in Cuba (Caribbean Sea) after the ingestion of a marine snail ( Turbo pica ) locally known as cigua [32]. The historical facts suggested the tropical and subtropical Pacific and Indian Ocean insular regions, the tropical Caribbean region, and the continental reefs to be endemic to ciguatera [27].
In recent years, there has been an increase in the frequency of reports of toxic and harmful benthic algal blooms, predominantly Gambierdiscus with the presence of Fukuyoa and Ostreopsis blooms, throughout the world [33,34][33][34]. The interest in Gambierdiscus blooms has been heightened because of increased awareness of the effects of CP on human health. A growth in species abundance has already been observed in subtropical and temperate regions with the threat of global warming expected to further exacerbate the situation [35,36,37,38][35][36][37][38]. However, a recent article by Hallegraeff et al. [39] suggested that intensified monitoring efforts and heightened aquaculture activities are responsible for these perceived increases in HABs events that are not underpinned to be expanded as an empirical assumption. Ciguatoxins are reported globally, being described from new sites in the Canary Islands, Indian Ocean, Japan, and Western Gulf of Mexico [8,40,41,42,43,44,45][8][40][41][42][43][44][45]. Until recently, the records of Gambierdiscus in the Indian Ocean were scarce and restricted to the western tropical region, whereas now its presence has been found in the Northern part of the ocean [42]. There is a paucity of information on ciguatera phenomena, including the occurrence of human poisonings, of toxins in seafood, and of the causative organisms, in the Indian Ocean in general, and the northern part in particular. In the present review, we gathered the published information on reported occurrences of Gambierdiscus and identified the research gaps related to its monitoring as a tool to manage this emerging hazard.
2. Ciguatera in the Indian Ocea
The Indian Ocean is the third largest ocean enclosing densely populated landmasses: In the North, India, Bangladesh, Burma, Thailand, Pakistan, Iran, and Oman among others. It also includes regional seas in the North such as the Arabian Sea and the adjacent Lakshadweep Sea, Aden Gulf in the Red Sea, the Gulf of Aqaba and Suez, the Bay of Bengal and the Andaman Sea, as well as the Gulf of Oman and the Persian Gulf further north. Countries from the Indian Ocean region have experienced unusual climatological conditions such as cyclones, El Niño-Southern Oscillation events, and coral reef bleaching during the past two decades [46,47,48][46][47][48]. Benthic microalgae are particularly influenced by these disturbances especially the coral mortality, which provides a good substrate for the formation of algal turfs and associated epiphytes [49]. The study conducted by Quod et al. [46] reported a significant increase in the CP causative organisms in comparison to those reported in 1980 [47], following the coral bleaching event in 1998, thus posing an incremental risk of HABs in the region and drawing detrimental consequences to the marine biodiversity and human health [50].
The unfavorable effects of increasing atmospheric levels of carbon dioxide (CO2) and other greenhouse gases in the Indian Ocean and its marginal seas are leading towards acidification of the marine environment [48,51,52,53][48][51][52][53]. Supporting evidence for increased CO2 sequestration was drawn from increased marine primary productivity over the past decade [51,54][51][54]. The eutrophication of Kuwait Bay and the nearby water bodies due to upwelling events is considered to be a predominant factor influencing the onset of algal blooms due to enriched nutrient conditions [54]. Other potential factors that have influenced the onset of blooms consist of coral bleaching, unusual variances in temperature, and calm conditions. Additional factors such as dust storms that carry micronutrients, domestic and industrial inputs, natural meteorological and oceanographic forcings, and the introduction of invasive species from ballast water discharge may all play a major role in the onset and expansion of HABs [41,45,55,56,57,58][41][45][55][56][57][58].
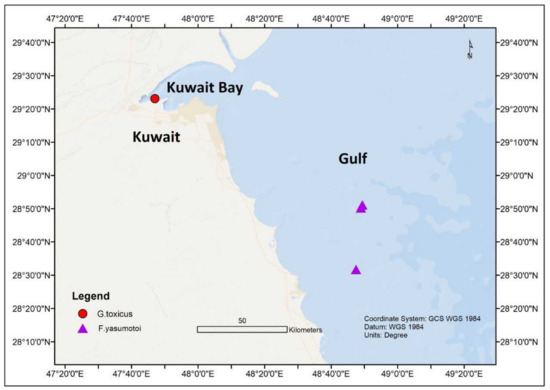
In the northern part of the Indian Ocean, Gambierdiscus species have been noticed in the marginal seas of the Indian Ocean, such as the Arabian Sea [41,42,45,68][41][42][45][59]; Red Sea [41,42,43,44,45][41][42][43][44][45]; Gulf of Aqaba [42]; and Manora Channel, Pakistan [69][60]. An assessment carried out in Kuwait (Persian Gulf) from November 2012 to March 2013 showed the presence of G. yasumotoi in the shallow lagoons of Qit’at Julai’ah and southern coastal waters of Kuwait at 1–3 m depth [68,70][59][61] ( Figure 4 ). The average seawater temperatures and salinity during this time were between 23.5–25 °C and 41.2–42.4 ppt, respectively. In another study, G. toxicus was also identified in Kuwait’s territorial water [41,45][41][45] ( Figure 4 ).
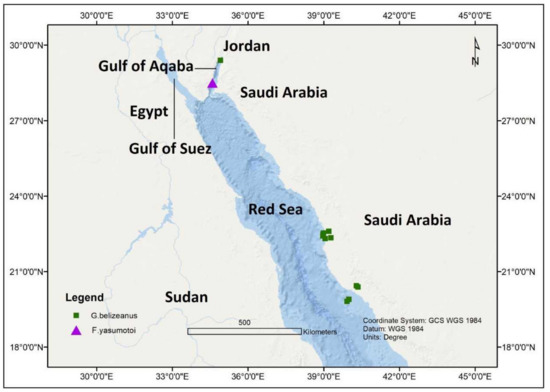
In an investigation from the Gulf of Aqaba, Jordan during October 2010, with a seawater temperature range of 24–25 °C and salinity of 40 ppt, G. belizeanus was reported from a depth of 1.5–2.0 m ( Figure 5 ). The toxigenic G. belizeanus was reported from the Red Sea, Saudi Arabian coast during February 2012 and May 2013 [43,44][43][44] at a sampling depth between 0.4–0.8 m, with seawater temperatures and salinity range of 28.9–38.4 °C and 36.1–38.4 ppt respectively. The oxygen saturation during this period was very variable between 1.73–32.1%.
3. Discussion
In the northern part of the Indian ocean, the cell numbers of Gambierdiscus from the central Red Sea, Saudi Arabian coast were <40 cells g −1 ww of algae [43,44][43][44]. Similarly, F. yasumotoi in Kuwaiti shores had an average cell density of 116.7 ± 47.5 cells g −1 of ww algae. The high biomass algae (HABs) were estimated through the Ocean Color Modis Algorithm (OC3M), Garver-Siegel-Maritorena Algorithm (GSM), Generalized Inherent Optical Property (GIOP) model [41,45][41][45]; these areas when sampled showed the presence of G. toxicus in a concentration of ~1000 cells per liter of seawater [41].
A variety of techniques are known to be used for ciguatera identification at a particular site. These include light microscopy (LM), scanning electron microscopy (SEM), DNA sequencing, restriction fragment length polymorphism (RFLP), quantitative polymerase chain reaction (qPCR), and metabarcoding [8,87][8][62]. While performing the morphological identification, the taxonomic key reported earlier [25] was followed by the research groups to confirm the species of Gambierdiscus found in the region. Earlier in the 1990s, SEM was used for the identification of G. toxicus from Reunion Islands [64][63]. SEM was also used recently to identify the species of G. toxicus ; G. belizeanus ; G. polynesiensis ; G. australes; and F. yasumotoi [69][60]. The morphological identification of Gulf strains of G. yasumotoi and G. belizeanus was based on both LM and SEM [42,43,44][42][43][44]. A description of morphological parameters of strains present in the Indian Ocean region is given in Table 2 .
| F. yasumotoi [12] | G. belizeanus [64] | G. toxicus [65] | G. polynesiensis [66] | G. australes [66] | |
|---|---|---|---|---|---|
| Cell depth | 54 to 73 µm | 59 to 73 µm | 71 to 96 µm | 65 to 87 µm | 60 to 82 µm |
| Cell width | 46 to 61 µm | 60 to 73 µm | 45 to 140 µm | 64 to 75 µm | 65 to 85 µm |
| Cell length | 49 to 70 µm | 47 to 51 µm | 50 to 150 µm | 57 to 75 µm | 76 to 93 µm |
| Cell shape | Globular | Round | Rounded/ellipsoid | Rounded/ellipsoid | Rounded/ellipsoid |
| Apical pore | Fish hook-shaped | Fish hook-shaped | Fish hook shaped | Fish hook shaped | Fish hook shaped |
| Thecal surface | Moderately aerated | Smooth | Smooth | ||
| Apical plate | elongated and teardrop-shaped | Ellipsoid | Ellipsoid | large triangular apical pore plate | broad ellipsoid apical pore plate |
| Hypotheca | Truncate-conical | Eight hypothecal plates | |||
| Epitheca | dome-shaped | Ellipsoid | Eleven plates | ||
| Sulcus | Short | ||||
| Chloroplast | elongated golden-brown chloroplasts were peripherally arranged | golden-brown drop-shaped chloroplasts radiating from the cell centre | |||
| Nucleus | large, oblong and located in the right posterior | large, oblong and crescent-shaped, and located in the right posterior cell part | Posteriorly situated nucleus | ||
| Cingulum | deeply excavated, descending, | Ascending narrow cingulum | Narrow and deep | ||
| Plate Formulae | Po, 3′, 7″, 6C, 8S, 5‴, 1p, 2⁗ | Po, 3′, 7″, 6C, 8S, 5‴, 1p et 2‴ | Po, 3′, 7″, 6C, 8S, 5‴, 1p et 2⁗ | ||
| Studied by | [42][60] | [42][43][44][60] | [67][60] | [60] | [60] |
Owing to the plasticity of morphological characteristics of some species of Gambierdiscus, the identification can be ambiguous if only morphology is used. Currently, molecular techniques are gaining popularity for the confirmation of species identified through microscopic methods. The only report on molecular identification of Gambierdiscus in the Arabian Gulf was by Catania [43] and her team [44]. The group amplified an 850 bp hypervariable (D8-D10) region of the larger subunit (LSU) of rRNA using primers FD8 and RB [11,25][11][25] through Sanger sequencing. A query of assembled nucleotides on the National Centre for Biotechnology and Informatics (NCBI) through the basic local alignment search tool (BLAST) resulted in a 100% match with G. belizeanus. Their (KY782637–KY782645) strains depicted a close resemblance with the Caribbean strain of G. belizeanus. The group also reported a 116 bp deletion at 493–609 position in one of the isolates, probably imparting the distinctiveness from other species of Gambierdiscus [95,96][68][69].
More diversity in clade IV and V including the species discovered from Indian Ocean may be due to a smaller number of samples owing to the recent discovery of these species. The presence of unclassified Fukuyoa sps. and Gambierdiscus ribotypes in these clades are suggestive of unexplored novel species. In 2016, at the 32nd session of the Codex Committee on Fisheries and Fishery Products, the Pacific Nations raised CP as an issue that is increasingly affecting the tropical and subtropical regions of the Pacific Ocean, the Indian Ocean, and the Caribbean Sea between the latitudes of 35° N and 35° S [8]. The strains found in the Indian Ocean and their adjoining seas lie near the strains commonly found in these high-risk regions. Probably, the interconnection between the water bodies and the ballast water is the major source of ciguatera infiltration in the Indian Ocean waters. Given this, extensive monitoring and risk management program should be developed for the unexplored regions and specifically in the Middle East region.
4. Conclusions
Ciguatera research in the Indian Ocean region is limited and fragmented. Although, there are reported cases of CP in the region. The most significant toxic fish in the region were barracuda (Sphyraenidae), amberjack (Seriola), grouper (Serranidae), snapper (Lutjanidae), po’ou (Labridae spp.), jack (Carangidae spp.) , trevally (Caranx spp.), wrasse (Labridae spp.), surgeonfish (Acanthuridae spp.), moray eel (Muraenidae spp.), roi (Cephalopholis spp.), parrotfish (Scaridae spp.), seabass, shark, and red snapper to name a few. Since the region exports fisheries, some of the fish originating from India and Sri Lanka in 2012, 2015, and 2016 were implicated in ciguatera poisoning in Europe.
A comprehensive survey of algal substrates in the region complemented with high throughput metabarcoding would provide insights into novel and undiscovered contributors of ciguatera. Although less in numbers, the presence of ciguatera causative organisms in the region cannot be ignored and their interaction with substrate and other microbial species is worthy of further investigation. The development of an early warning system for HABs is very much the need of the hour.
References
- Senthilkumaran, S.; Meenakshisundaram, R.; Michaels, A.; Suresh, P.; Thirumalaikolundusubramanian, P. Cardiovascular complications in ciguatera fish poisoning: A wake-up call. Heart Views Off. J. Gulf Heart Assoc. 2011, 12, 166.
- Albinali, H. Ciguatera fish poisoning. Heart Views 2011, 12, 165.
- Accoroni, S.; Romagnoli, T.; Colombo, F.; Pennesi, C.; Di Camillo, C.G.; Marini, M.; Battocchi, C.; Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; et al. Ostreopsis cf. ovata bloom in the northern Adriatic Sea during summer 2009: Ecology, molecular characterization and toxin profile. Mar. Pollut. Bull. 2011, 62, 2512–2519.
- Chinain, M.; Darius, H.; Ung, A.; Cruchet, P.; Wang, Z.; Ponton, D.; Laurent, D.; Pauillac, S. Growth and toxin production in the ciguatera-causing dinoflagellate Gambierdiscus polynesiensis (Dinophyceae) in culture. Toxicon 2010, 56, 739–750.
- Chinain, M.; Gatti, C.M.i.; Darius, H.T.; Quod, J.P.; Tester, P.A. Ciguatera poisonings: A global review of occurrences and trends. Harmful Algae 2021, 102, 101873.
- Friedman, M.; Fleming, L.; Fernandez, M.; Bienfang, P.; Schrank, K.; Dickey, R.; Bottein, M.; Backer, L.; Ayyar, R.; Weisman, R. Ciguatera fish poisoning: Treatment, prevention and management. Mar. Drugs 2008, 6, 456–479.
- Fleming, L.; Baden, D.; Bean, J.; Weisman, R.; Blythe, D. Marine Seafood Toxin Diseases: Issues in Epidemiology & Community Outreach; National Institute of Environmental Health Sciences: Durham, NC, USA, 1998.
- WHO, F.A. Report of the Expert Meeting on Ciguatera poisoning: Rome, 19–23 November 2018; Food Safety and Quality Series; World Health Organization: Rome, Italy, 2020.
- Parsons, M.; Richlen, M. An overview of ciguatera fish poisoning in the Bahamas. In The 15th Symposium on the Natural History of the Bahamas; Gerace Research Centre San Salvador Bahamas; A & A Printing Inc.: Tampa, Florida, USA, 2016.
- Chinain, M.; Gatti, C.; Roué, M.; Darius, H. Ciguatera-causing dinoflagellates in the genera gambierdiscus and fukuyoa: Distribution, ecophysiology and toxicology. In Dinoflagellates: Morphology, Life History and Ecological Significance; Subba Rao, D.V., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2020; pp. 405–457.
- Litaker, R.W.; Vandersea, M.W.; Faust, M.A.; Kibler, S.R.; Nau, A.W.; Holland, W.C.; Chinain, M.; Holmes, M.J.; Tester, P.A. Global distribution of ciguatera causing dinoflagellates in the genus Gambierdiscus. Toxicon 2010, 56, 711–730.
- Holmes, M. Gambierdiscus yasumotoi sp. nov.(Dinophyceae), a toxic benthic dinoflagellate from southeastern Asia. J. Phycol. 1998, 34, 661–668.
- Kashiwada, S.; Ishikawa, H.; Miyamoto, N.; Ohnishi, Y.; Magara, Y. Fish test for endocrine-disruption and estimation of water quality of Japanese rivers. Water Res. 2002, 36, 2161–2166.
- Nishimura, T.; Sato, S.; Tawong, W.; Sakanari, H.; Uehara, K.; Shah, M.; Suda, S.; Yasumoto, T.; Taira, Y.; Yamaguchi, H. Genetic diversity and distribution of the ciguatera-causing dinoflagellate Gambierdiscus spp.(Dinophyceae) in coastal areas of Japan. PLoS ONE 2013, 8, e60882.
- Rhodes, L.; Gimenez, P.; Smith, K.; Harwood, T. Gambierdiscus cf. yasumotoi (Dinophyceae) isolated from New Zealand’s sub-tropical northern coastal waters. N. Z. J. Mar. Freshw. Res. 2014, 48, 303–310.
- Casabianca, S.; Perini, F.; Casabianca, A.; Battocchi, C.; Giussani, V.; Chiantore, M.; Penna, A. Monitoring toxic Ostreopsis cf. ovata in recreational waters using a qPCR based assay. Mar. Pollut. Bull. 2014, 88, 102–109.
- Cohu, S.; Thibaut, T.; Mangialajo, L.; Labat, J.P.; Passafiume, O.; Blanfune, A.; Simon, N.; Cottalorda, J.M.; Lemee, R. Occurrence of the toxic dinoflagellate Ostreopsis cf. ovata in relation with environmental factors in Monaco (NW Mediterranean). Mar. Pollut. Bull. 2011, 62, 2681–2691.
- Parsons, M.L.; Brandt, A.L.; Ellsworth, A.; Leynse, A.K.; Rains, L.K.; Anderson, D.M. Assessing the use of artificial substrates to monitor Gambierdiscus populations in the Florida Keys. Harmful Algae 2017, 68, 52–66.
- Yasumoto, T.; Nakajima, I.; Bagnis, R.; Adachi, R. Finding of a dinoflagellate as a likely culprit of ciguatera. Bull. Jpn. Soc. Sci. Fish. 1977, 43, 1021–1026.
- Yasumoto, T.; Inoue, A.; Bagnis, R. Ecological survey of a toxic dinoflagellate associated with ciguatera. In Toxic Dinoflagellate Blooms; Taylor, D.L., Seliger, H.H., Eds.; Elsevier North Holland, Inc.: New York, NY, USA, 1979; pp. 221–224.
- Yasumoto, T.; Inoue, A.; Ochi, T.; Fujimoto, K.; Oshima, Y.; Fukuyo, Y.; Adachi, R.; Bagnis, R. Environmental studies on a toxic dinoflagellate responsible for ciguatera. Bull. Jpn. Soc. Sci. Fish. 1980, 46, 1397–1404.
- Ballantine, D.L.; Tosteson, T.R.; Bardales, A.T. Population dynamics and toxicity of natural populations of benthic dinoflagellates in southwestern Puerto Rico. J. Exp. Mar. Biol. Ecol. 1988, 119, 201–212.
- Lobel, P.S.; Anderson, D.M.; Durand-Clement, M. Assessment of Ciguatera Dinoflagellate Populations: Sample Variability and Algal Substrate Selection. Biol. Bull. 1988, 175, 94–101.
- Bomber, J.W.; Rubio, M.G.; Norris, D.R. Epiphytism of dinoflagellates associated with the disease ciguatera: Substrate specificity and nutrition. Phycologia 1989, 28, 360–368.
- Litaker, R.; Vandersea, M.; Faust, M.; Kibler, S.; Chinain, M.; Holmes, M.; Holland, W.; Tester, P. Taxonomy of Gambierdiscus including four new species, Gambierdiscus caribaeus, Gambierdiscus carolinianus, Gambierdiscus carpenteri and Gambierdiscus ruetzleri (Gonyaulacales, Dinophyceae). Phycologia 2009, 48, 344–390.
- Rhodes, L.; Smith, K.; Murray, J.; Nishimura, T.; Finch, S. Ciguatera fish poisoning: The risk from an Aotearoa/New Zealand perspective. Toxins 2020, 12, 50.
- Biotoxins, F.M. FAO Food and Nutrition Paper 80; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004.
- De Rota, F. URDANETA¿ CIENTÍFICO? Institute de Historia and Cultura Naval: Madrid, Spain, 2009; 38p.
- Pearn, J. Around the rim: The role of surgeons in discovery and research in the Pacific rim. Part I. Surgeons in the Pacific: Expeditioners and expedition leaders. Aust. New Zealand J. Surg. 1994, 64, 38–44.
- Lee, C. Fish poisoning with particular reference to ciguatera. J. Trop. Med. Hyg. 1980, 83, 93–97.
- Silva, S. Contribution al’etude du microplankton de dakar et des regions maritimes voisines. Bull. Inst. Fr. Afr. Noire. Ser. A. Sci. Nat. 1956, 18, 335–371.
- Guzman-Perez, S.; Park, D. Ciguatera toxins: Chemistry and detection. Food Sci. Technol. N.Y. Marcel Dekker 2000, 401–418.
- Tester, P.A.; Litaker, R.W.; Berdalet, E. Climate change and harmful benthic microalgae. Harmful Algae 2020, 91, 101655.
- Landrigan, P.J.; Stegeman, J.J.; Fleming, L.E.; Allemand, D.; Anderson, D.M.; Backer, L.C.; Brucker-Davis, F.; Chevalier, N.; Corra, L.; Czerucka, D.; et al. Human Health and Ocean Pollution. Ann. Glob. Health 2020, 86, 1–64.
- Kibler, S.; Litaker, R.; Holland, W.; Vandersea, M.; Tester, P. Growth of eight Gambierdiscus (Dinophyceae) species: Effects of temperature, salinity and irradiance. Harmful Algae 2012, 19, 1–14.
- Gingold, D.B.; Strickland, M.J.; Hess, J.J. Ciguatera fish poisoning and climate change: Analysis of National Poison Center Data in the United States, 2001–2011. Environ. Health Perspect. 2014, 122, 580–586.
- Kibler, S.R.; Tester, P.A.; Kunkel, K.E.; Moore, S.K.; Litaker, R.W. Effects of ocean warming on growth and distribution of dinoflagellates associated with ciguatera fish poisoning in the Caribbean. Ecol. Model. 2015, 316, 194–210.
- Yong, H.L.; Mustapa, N.I.; Lee, L.K.; Lim, Z.F.; Tan, T.H.; Usup, G.; Gu, H.; Litaker, R.W.; Tester, P.A.; Lim, P.T. Habitat complexity affects benthic harmful dinoflagellate assemblages in the fringing reef of Rawa Island, Malaysia. Harmful Algae 2018, 78, 56–68.
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Bottein, M.-Y.D.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Commun. Earth Environ. 2021, 2, 117.
- Rhodes, L.; Smith, K.; Munday, R.; Selwood, A.; McNabb, P.; Holland, P.; Bottein, M. Toxic dinoflagellates (Dinophyceae) from Rarotonga, Cook Islands. Toxicon 2010, 56, 751–758.
- Uddin, S.; Sultan, M.; Behbehani, M.; Al-Rashed, W.; Al-Shamroukh, D.; Al-Khabbaz, A.; Al-Yaegoub, A.; Al-Bahloul, M. A Remote Sensing-Based Early Warning System for Algal Blooms in Kuwait Bay and Coastal Waters; Project EM033C, Final Report, KISR12320; Kuwait Institute for Scientific Research, 2014; 145p.
- Saburova, M.; Polikarpov, I.; Al-Yamani, F. New records of the genus Gambierdiscus in marginal seas of the Indian Ocean. Mar. Biodivers. Rec. 2013, 6, e91.
- Catania, D. The Prevalence of Benthic Dinoflagellates Associated with Ciguatera in the Central Red Sea. M.Sc. Thesis, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia, 2012; 40p.
- Catania, D.; Richlen, M.; Mak, Y.; Morton, S.; Laban, E.; Xu, Y.; Anderson, D.; Chan, L.; Berumen, M. The prevalence of benthic dinoflagellates associated with ciguatera fish poisoning in the central Red Sea. Harmful Algae 2017, 68, 206–216.
- Manche, C. A Remote Sensing Based Early Warning System for Algal Blooms in Kuwait Bay and Coastal Waters. Master’s Thesis, Western Michigan University, Kalamazoo, MI, USA, 2014. Available online: https://scholarworks.wmich.edu/masters_theses/546 (accessed on 1 June 2021).
- Quod, J.-P.; Turquet, J.; Conejero, S.; Ralijaona, C. Ciguatera risk assessment in the Indian Ocean following the 1998 coral bleaching event. Coral Reef Degrad. Indian Ocean. Status Rep. 2000, 166–168.
- Bagnis, R. L’ichtyosarcotoxisme de type ciguatera: Phénomène complexe de biologie marine et humaine. Oceanol. Acta 1981, 4, 375–387.
- Behbehani, M.; Uddin, S.; Dupont, S.; Sajid, S.; Al-Musalam, L.; Al-Ghadban, A. Response of corals Acropora pharaonis and Porites lutea to changes in pH and temperature in the Gulf. Sustainability 2019, 11, 3156.
- Reguera, B.; Alonso, R.; Moreira, A.; Méndez, S.; Dechraoui-Bottein, M.-Y. Guide for Designing and Implementing a Plan to Monitor Toxin-Producing Microalgae. In Intergovermental Oceanographic Commission Manuals and Guides, 2nd ed.; UNESCO: Paris, France; IAEA: Vienna, Austria, 2016; 66p, Available online: http://hdl.handle.net/11329/304 (accessed on 1 June 2021).
- Jean Turquet, J.-P.Q.; Ten-Hage, L.; Dahalani, Y.; Wendling, B. Example of a Gambierdiscus toxicus flare-up following the 1998 coral bleaching event in Mayotte Island (Comoros, south-west Indian Ocean). In Proceedings of the Harmful Algal Blooms 2000: Proceedings of the Ninth International Conference on Harmful Algal Blooms, Hobart, Australia, 7–11 February 2000.
- Uddin, S.; Gevao, B.; Al-Ghadban, A.; Nithyanandan, M.; Al-Shamroukh, D. Acidification in Arabian Gulf–Insights from pH and temperature measurements. J. Environ. Monit. 2012, 14, 1479–1482.
- Al-Musalam, L.; Uddin, S. Effect of Ocean Acidification on Growth and Abundance of Penaeus Semisulcatus in the Northern Arabian Gulf; Annual Progress Report No. 1(EM078C); Kuwait Insitute for Scientific Research: Kuwait City, Kuwait, 2018; 27p.
- Al-Musallam, L.; Uddin, S.; Al-Dakkor, S.; Kumar, V. Effect of ocean acidification and ocean warming on the growth and survival of Penaeus semisulcatus Post-Larvae. J Earth Env. Sci 2019, 3.
- Elkadiri, R.; Manche, C.; Sultan, M.; Al-Dousari, A.; Uddin, S.; Chouinard, K.; Abotalib, A.Z. Development of a Coupled Spatiotemporal Algal Bloom Model for Coastal Areas: A Remote Sensing and Data Mining-Based Approach. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2016, 9, 5159–5171.
- Heil, C.A.; Glibert, P.M.; Al-Sarawi, M.A.; Faraj, M.; Behbehani, M.; Husain, M. First record of a fish-killing Gymnodinium sp. bloom in Kuwait Bay, Arabian Sea: Chronology and potential causes. Mar. Ecol. Prog. Ser. 2001, 214, 15–23.
- Glibert, P.; Landsberg, J.; Evans, J.; Al-Sarawi, M.; Faraj, M.; Al-Jarallah, M.; Haywood, A.; Ibrahem, S.; Klesius, P.; Powell, C. A fish kill of massive proportion in Kuwait Bay, Arabian Gulf, 2001: The roles of bacterial disease, harmful algae, and eutrophication. Harmful Algae 2002, 1, 215–231.
- Walsh, J.; Steidinger, K. Saharan dust and Florida red tides: The cyanophyte connection. J. Geophys. Res. Ocean. 2001, 106, 11597–11612.
- Richlen, M.L.; Morton, S.L.; Jamali, E.A.; Rajan, A.; Anderson, D.M. The catastrophic 2008–2009 red tide in the Arabian gulf region, with observations on the identification and phylogeny of the fish-killing dinoflagellate Cochlodinium polykrikoides. Harmful Algae 2010, 9, 163–172.
- Saburova, M.; Igor, P.; Al-Yamani, F. Gambierdiscus in Kuwait. Harmful Algae News 2013, 47, 22–23.
- Munir, S.; Siddiqui, P.; Morton, S.L. The occurrence of the ciguatera fish poisoning producing dinoflagellate genus Gambierdiscus in Pakistan waters. Algae 2011, 26, 317–325.
- Polikarpov, I.; Saburova, M.; Al-Yamani, F. Diversity and distribution of winter phytoplankton in the Arabian Gulf and the Sea of Oman. Cont. Shelf Res. 2016, 119, 85–99.
- Roué, M.; Smith, K.; Sibat, M.; Viallon, J.; Henry, K.; Ung, A.; Biessy, L.; Hess, P.; Darius, H.; Chinain, M. Assessment of Ciguatera and Other Phycotoxin-Related Risks in Anaho Bay (Nuku Hiva Island, French Polynesia): Molecular, Toxicological, and Chemical Analyses of Passive Samplers. Toxins 2020, 12, 321.
- Quod, J.; Turquet, J. Ciguatera in Reunion Island (SW Indian Ocean): Epidemiology and clinical patterns. Toxicon 1996, 34, 779–785.
- Faust, M. Observation of sand-dwelling toxic dinoflagellates (Dinophyceae) from widely differing sites, including two new species. J. Phycol. 1995, 31, 996–1003.
- Adachi, R.; Fukuyo, Y. The thecal structure of a marine toxic dinoflagellate Gambierdiscus toxicus gen. et sp. nov. collected in a ciguatera endemic area. Bull. Jpn. Soc. Sci. Fish. 1979, 45, 67–71.
- Chinain, M.; Faust, M.A.; Pauillac, S. Morphology and molecular analyses of three toxic species of Gambierdiscus (Dinophyceae): G. pacificus sp. nov., G. australes, sp. nov. and G. polynesiensis, sp. nov. J. Phycol. 1999, 35, 1282–1296.
- Lugomela, C. Autecology of the Toxic Dinoflagellate Gambierdiscus toxicus Adachi et Fukyo (Dinophyceae) in Central Coastal Areas of Tanzania. West. Indian Ocean J. Mar. Sci. 2006, 5, 213–221.
- Xu, Y.; Richlen, M.L.; Morton, S.L.; Mak, Y.L.; Chan, L.L.; Tekiau, A.; Anderson, D.M. Distribution, abundance and diversity of Gambierdiscus spp. from a ciguatera-endemic area in Marakei, Republic of Kiribati. Harmful Algae 2014, 34, 56–68.
- Dai, X.; Mak, Y.; Lu, C.; Mei, H.; Wu, J.; Lee, W.; Chan, L.; Lim, P.; Mustapa, N.; Lim, H. Taxonomic assignment of the benthic toxigenic dinoflagellate Gambierdiscus sp. type 6 as Gambierdiscus balechii (Dinophyceae), including its distribution and ciguatoxicity. Harmful Algae 2017, 67, 107–118.
