Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Anna Atlante.
Mitochondria are complex intracellular organelles traditionally identified as the powerhouses of eukaryotic cells due to their central role in bioenergetic metabolism. The intercellular transport of mitochondria, defined as horizontal mitochondrial transfer, can occur in mammalian cells both in vitro and in vivo, and in physiological and pathological conditions. Mitochondrial transfer can provide an exogenous mitochondrial source, replenishing dysfunctional mitochondria, thereby improving mitochondrial faults or, as in in the case of tumor cells, changing their functional skills and response to chemotherapy.
- mitochondria
- bioenergetics
- oxidative phosphorylation
- intercellular mitochondria trafficking
- extracellular mitochondria
1. Introduction
Mitochondria are complex intracellular organelles long identified as the cellular power plants due to their vital role in oxidative energy metabolism. Acting as central metabolic hubs, mitochondria rapidly adapt to different environmental cues and metabolic alterations to meet the bioenergetics demands of the cell, recently defined as mitochondrial plasticity [1]. Owing to their highly plastic nature, mitochondria constitute a dynamic network of signaling organelles with multifunctional key roles in cell metabolism, proliferation and survival [2]. Notably, a variety of pre-clinical and clinical studies have demonstrated that metabolic and/or genetic mitochondrial alterations are involved in the pathogenesis of a large number of diseases, including cancer [3,4][3][4].
Mitochondria are highly interconnected entities ongoing fusion and fission events [5], with the capability to dynamically redesign their morphology as well as to move within the cell, thus supporting critical energy power needs [6]. Homeostasis of the mitochondrial pool is guaranteed by selective removal of mitochondria through mitophagy and by biogenetic mitochondrial supply, thus keeping an active and dynamic mitochondrial network within the cell [7]. In particular, in neurons, anterograde mitochondrial transfer from the cell body to the axonal extensions of the cell, or retrograde moving from axon to cell body can allow the removal of injured mitochondria or the restoration of healthy mitochondria. [8]. In invasive cancer cells, mitochondria relocate to the leading edge of the cells to provide fuel for their movement [9,10][9][10].
Emerging evidence is revealing that the dynamic nature of mitochondria can go beyond cell boundaries, allowing for their translocation between mammalian cells, radically challenging the hitherto known concepts of intracellular segregation of mitochondria and mitochondrial DNA (mtDNA) inheritance [11]. Their signaling role can extend to intercellular communication, showing that the mitochondrial genome and even entire mitochondria are indeed mobile and can mediate the transfer of information between cells. This mobile transfer task of mitochondria and mtDNA has been recently termed “momioma” to indicate all the “mobile functions of mitochondria and mitochondrial genome” [12]. Mitochondrial intercellular transfer promotes the integration of mitochondria into the endogenous mitochondrial network of recipient cells, contributing to changes in their bioenergetic status and other functional skills of receiver cells, not only in vitro but also in vivo [13]. Furthermore, the horizontal transfer of mitochondrial genes can lead to serious implications in the pathophysiology of mitochondrial dysfunction [14].
Although the physiological relevance of this phenomenon is still a matter of debate, several in vitro and in vivo studies have shown how the transfer of mitochondria among cells is able to recover mitochondrial respiratory defects in recipient cells, rescuing and regulating signaling, proliferation or resistance to chemotherapy, also functioning as means of tissue revitalization [15].
2. Modes and Cellular Structures Mediating Intercellular Mitochondrial Transfer
The molecular mechanisms through which cells containing dysfunctional mitochondria acquire new mitochondria from other cells and the signaling pathways regulating this process remains yet poorly understood. The cells likely have developed mechanisms to trigger the transfer in response to the injury signals emanating from the recipient cell. However, the molecular signals initiating this type of crosstalk are yet unknown.
Several structures are involved in transcellular mitochondria transfer [16] (the main forms are depicted in Figure 1), among which tunneling nanotubes (TNTs) represent the main cellular system mediating intercellular mitochondrial translocation. Other modes of transfer have been identified, including membrane micro-vesicles, gap junctions, cell fusion or mitochondrial expulsion. Mitochondrial transfer through these different structures can lead to different functional outcomes for the recipient cells, i.e., functional mitochondrial acquisition, immune activation or trans-mitophagy [14]. Therefore, a clear understanding of the mechanisms that mediate mitochondrial transfer will shed light on how this process is regulated and can be exploited for therapeutic purposes.
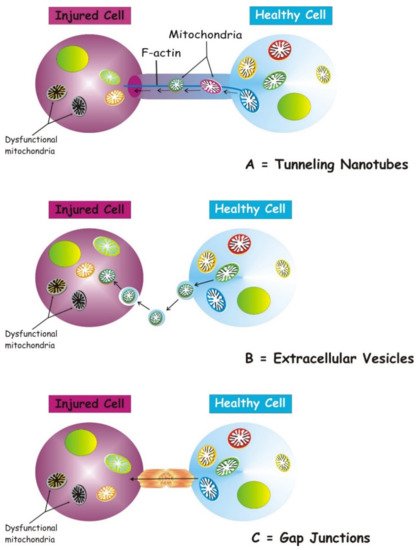
Figure 1. Three main forms of intercellular communication related to transcellular mitochondrial transfer. Under different injury signaling, three main forms of intercellular communication related to mitochondrial transcellular transfer can be formed: tunneling nanotubes (A), extracellular vesicles (B), and gap junctions. (C). For details see the text.
TNTs are nanotubular structures generated through formation of cell membrane protrusions that attach to the target cell. The membrane of each cell extends to fuse together, thus forming a firmly connected bridge suspended in the extracellular space [17]. TNTs allow unidirectional and bidirectional transport of a variety of components, including small molecules, proteins, organelles, and even viral particles [18,19,20][18][19][20].
The discovery of TNTs in 2004 appeared as an innovative cell-to-cell communication mechanism, demonstrating the ability of mammalian cells to exchange organelles with other cells [21]. TNTs are dynamic structures forming de novo in a few minutes and with a half-life ranging from a few minutes to several hours [21,22][21][22]. TNTs have a skeleton composed mainly of F-actin and transport proteins that assist the active transport of cargoes and mitochondria along these structures [23]. TNTs act as channels for mitochondrial transfer between various cells. In particular, under conditions of oxidative stress, the intracellular expression of p53 is upregulated and the protein kinase B-phosphoinositide 3-kinase-mechanistic target of rapamycin (AKT-PI3K-mTOR) signaling pathway is activated, leading to the formation of TNTs between stressed and unstressed cells mediating the transcellular transport of several organelles, including ER, Golgi, endosomes and mitochondria [24]. In the bone marrow microenvironment of multiple myeloma, TNT-mediated transcellular transfer of mitochondria from neighboring nonmalignant bone marrow stromal cells to multiple myeloma cells supports oxidative phosphorylation of multiple myeloma cells and is dependent on the expression of CD38 [25]. TNTs also form between tumor cells and have been implicated in their survival and drug resistance [26,27][26][27].
In neurons, the molecular motor myosin X is also required for TNT formation [28]. A study picked out the participation of an extracellular protein, S100A4, and its receptor RAGE (Receptor for Advanced Glycation End Product) in managing TNT growth direction in the nervous tissue [29]. Under oxidative stress, hippocampal neurons and astrocytes initiated the formation of TNTs after p53-mediated activation of caspase-3. S100A4 cleavage by activated caspase-3 formed a gradient of low levels of S100A4 in initiating damaged cells towards a higher concentration in receiving cells, i.e., astrocytes [29]. The results of this study disclose that injured neurons transfer cellular contents to astrocytes, scattering the danger signals and inducing mitochondrial transfer. TNT-mediated mitochondrial transfer can act as a survival way for cells under stress, for example, by saving damaged ischemic cells [30], by protecting the alveolar epithelium from injury [31] and repairing the tissues [32,33,34][32][33][34].
2.1. Mitochondrial Transfer via Extracellular Vesicles
A heterogeneous population of vesicles (ranging from 40 to 1000 nm) are generally released from the intracellular to the extracellular environment. These vesicles are denoted as extracellular vesicles (EVs). They include exosomes (30–100 nm in diameter), micro-vesicles (MVs) (100 nm to 1 μm in diameter) and apoptotic bodies (1–2 μm) depending on their source and molecular structure [35]. Apoptotic bodies are the least studied due to their rapid elimination by phagocytic cells [36]. Exosomes and micro-vesicles were originally defined as vesicles to eliminate ancient proteins in immune cells and reticulocytes [36], but, more recently, it has been shown that they are released from nearly all cell types and considered responsible for communication between cells in a variety of pathophysiological events [37].
EVs containing lipids, proteins, RNA and mitochondria represent a competent mode to transport functional loads from one cell to another [38]. This discovery has introduced a new mode of interactions to an already multifaceted communication network and a new signal transmission mechanism [39].
Although the mechanisms by which mitochondrial proteins or mtDNA are loaded into EVs are still unknown, surely mitochondrial components have been detected in EVs. If smaller EVs such as exosomes can mainly carry small RNAs [40], but genomic DNA and mtDNA have also been detected [41[41][42],42], larger EVs such as MVs can enclose even entire mitochondria [43]. MVs carrying mitochondria can be secreted by different cell types, as observed in astrocytes, neurons and mesenchymal stem cells [41,44,45,46,47][41][44][45][46][47] and transferred into epithelial cells, immune cells, astrocytes and neurons [11]. The mitochondrial transfer does not always keep damaged cells, but also works to recover organelles into other cells by a transcellular degradation process [44,45][44][45]. Furthermore, Morrison et al. showed that mesenchymal stromal cells (MSCs) modulate macrophages, improving their respiration and phagocytic activity in clinically relevant lung injury models by EVs containing mitochondria [47]. Importantly, these exosome-mediated transferred mitochondria co-localize with the mitochondrial network and produce reactive oxygen species (ROS) within the recipient T cells [46].
2.2. Transfer via Gap Junction Channels
Gap junction channel (GJC) is a structure joining the cytoplasm of two separate cells. GJCs allow the passive transport of nutrients, metabolites, second messengers, cations, anions and also mitochondria [31,48,49][31][48][49]. Cx43 is a connexin that participates in the transcellular transfer of mitochondria. In a model of lipopolysaccharide (LPS)-induced acute lung injury, bone marrow stromal cells (BMSCs) transport mitochondria to the damaged alveolar epithelium, a process based on Ca2+ exchange between the two cells through CX43-GJCs [31]. Recently, it was shown that the transfer of mitochondria from BMSCs to hematopoietic stem cells (HSCs) is a timely physiological event in the mammalian response to acute bacterial infection [47]. Mechanistically, oxidative stress was found to regulate the opening of connexin channels in a PI3K-mediated system, the activation of which allowed the transfer of mitochondria from BMSCs to HSCs [46]. Cx43-GJCs participate in the intercellular exchange of ROS [50] and a direct mitochondrial transfer via Cx43 GJC has been hypothesized [16], which could represent a mechanism for TNT formation and intercellular transport of mitochondria.
2.3. Mitochondrial Transfer via Other Routes: Mitochondrial Extrusion and Cell Fusion
In addition to TNTs, EVs and Cx43-GJCs, representing the main routes that mediate transcellular transfer of mitochondria, other potential mechanisms for mitochondrial transfer from one cell to another are the mitochondrial extrusion and the cytoplasmic fusion.
The mitochondrial extrusion allows the release of mitochondria or mitochondrial components from cells under specific conditions during which mitochondria become inadequate to remain in cells. Retaining damaged mitochondria can produce large quantities of ROS [50] and under such circumstances, cells tend to dispose mitochondria extrusion into the intercellular space [51]. Naked mitochondria or mitochondrial components can also be extruded and internalized without carrier, through processes of exocytosis and endocytosis [15]. Mitochondrial extrusion takes place not only in vitro but also in vivo. Few studies have shown the release of naked or encapsulated mitochondria into the extracellular environment. Nakajima et al. confirmed in a mouse model the release of naked mitochondria into the intercellular space after an injection of anti-Fas antibodies [52]. In response to this treatment, the cytoplasmic vacuoles englobed the fragmented mitochondria and extruded them from the apoptotic hepatocytes. Similarly, activated platelets released respiration-competent mitochondria, as either free organelles or mitochondria engulfed within microparticles [52].
Cytoplasmic fusion is a common phenomenon in which the membrane of two or more cells fuse together, sharing the organelles, when injury and inflammation may trigger this process [53]. In particular, cell fusion regulates the potential of stem cells, playing a significant role in regeneration and oncogenesis [54]. Mitochondrial transfer takes part in this process [16].
3. Intercellular Mitochondrial Transfer in Different Pathophysiological Conditions
3.1. Transcellular Mitochondrial Transfer in Nervous Cells
In the central nervous system (CNS), mitochondrial transfer constitutes an important form of intercellular crosstalk contributing to the homeostasis and neuroprotection of CNS [45]. The transfer of mitochondria among nervous cells participates in the cellular and tissue defense against CNS damage, performing a relevant function in recovery after injury [45]. In the CNS, astrocytes perform a wide range of functions, including the regulation of neurodevelopment, neurotransmission and metabolism (reviewed in [14]). Emerging studies show that the neuroprotective functions of astrocytes may comprise the transfer of mitochondria from these cells to damaged neurons [45,55,56][45][55][56]. Under physiological conditions, astrocytes defend neurons, counteracting oxidative stress and excitotoxicity and ensuring neurotrophic support [57,58][57][58]. In the context of injury, astrocytes can transfer healthy mitochondria to axons [45,59][45][59]. Recently, a study of Cheng et al. in co-culture systems of human-induced pluripotent stem cells (iPSC) showed that iPSC-derived astrocytes can transfer mitochondria to neurons, recovering dopaminergic neuronal toxicity [60]. The neuroprotective function of astrocyte-derived mitochondria transfer to neurons has been highlighted in other studies of neurotoxicity; for instance, English et al. demonstrated, in their in vitro work on a co-culture of cisplatin-treated neurons with astrocytes, that neurons that had received mitochondria from astrocytes showed improved neuronal survival, reinstated neuronal mitochondrial membrane potential and normalized neuronal calcium dynamics, underlining the relevance of transcellular mitochondrial transfer [61]. Notably, under stress conditions, astrocytes may interrupt their protective function and release danger signaling factors, such as inflammatory cytokines, thus damaging neurons (reviewed in [14]).
In broader terms, there is growing evidence revealing that like in the whole CNS, transcellular transfer of mitochondria can have significant relevance and multifunctional roles in various physio-pathological contexts:
-
The transfer of mitochondria from neurons to astrocytes can activate a process known as trans-mitophagy, allowing cells to degrade dysfunctional mitochondria [45];
-
The transfer of mitochondria from hematopoietic stem and progenitor cells to neurons can improve their mitochondrial functional efficiency [14].
3.2. Mitochondrial Transfer in Dysfunctional Mitochondria-Related Neurodegenerative Disorders: Therapeutic Use of Exogenous Mitochondria for Alzheimer’s and Parkinson’s Diseases
Pathogenesis of neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases, involves dysfunction of mitochondria [64,65][64][65].
Alzheimer’s disease (AD) is the most common neurodegenerative disorder, characterized by a progressive failure in cognitive function due to progressive loss of neurons in forebrain and other brain areas [66]. Mitochondrial dysfunction has been established as an early and prominent feature of the disease [67]. The multiform, and even opposed, modes involving mitochondrial dysfunction in AD pathophysiology and their complex regulation make the aim of targeting mitochondrial deficits very difficult. Nitzan and coworker’s experimental strategy aimed at overcoming this limitation by using active and functional mitochondria, thereby allowing mitochondria to act as whole organelles rather than targeting only one of their dysfunctional tasks. The results of their analysis suggest that transfer of functionally active mitochondria, aimed at efficiently mimicking mitochondrial function, is beneficial to treat AD deficits, correcting cognitive deficits, brain pathology and mitochondrial defects in an AD mouse model [68]. In this recent in vivo study, the effect of transferring active intact mitochondria was investigated, by treating AD-mice (amyloid, intracerebroventricularly injected) intravenously (IV) with fresh human isolated mitochondria. Fourteen days after mitochondrial transplantation, AD-mice treated with exogenous mitochondria showed significantly improved cognitive performances almost comparable to those of untreated control mice [68]. A significant recovery in neuronal loss and reduced gliosis were also detected in the hippocampus of treated mice respect to untreated AD-mice. Increased citrate synthase and cytochrome c oxidase activities were measured in mitochondria-treated AD-mice, reaching activity values close to untreated control mice [68]. Increased mitochondrial activity was also detected in the liver of mitochondria-treated mice. No toxicity associated with the treatment was detected. Therefore, mitochondrial transfer could offer a novel therapeutic approach for AD treatment.
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, characterized by the selective loss of dopaminergic neurons of the substantia nigra pars compacta (SNc) with motor and nonmotor symptoms [69]. The main histopathological marker of PD is the presence in neurons of α-synuclein (α-syn) protein aggregates forming in inclusion bodies, indicated as Lewy bodies [70]. α-Syn is principally expressed pre-synaptically and there is evidence of the existence of α-syn transfer from neurons to neuronal and non-neuronal cells in vitro, indicating that α-syn pathology propagates between anatomically adjacent brain regions by an intercellular transfer mode [71]. Mitochondrial dysfunction is widely recognized as a common central pathway involved in the pathogenetic processes of sporadic and genetic PD (reviewed in [72]). Dysfunctional mitochondria are constant presences in PD [73]; moreover, α-syn can be located at mitochondrial membranes and its aggregation can be related to mitochondrial dysfunction in PD [74]. Increased ROS levels resulting from reduced efficiency in the electron transport chain activity are involved in the formation of α-syn aggregates and neuronal loss [75].
Increasing evidence suggests that astrocytes have a relevant part in the progression of PD (reviewed in [72]). A recent work presented clear evidence that transneuronal mitophagy occurs in vivo in PD models [76]. In PD models, astrocytes are primarily responsible for clearance of damaged mitochondria—a functional role of considerable relevance in the context of PD associated to mutations of Parkin and PINK1 [72]. Notably, PINK1 activity was recently predominantly found in astrocytes while almost absent in neurons [77]. Exogenous supplementation of mitochondria to damaged regions may be a potential and innovative therapeutic strategy for the treatment of PD, as shown in the in vivo work of Chang et al., demonstrating that injection of mitochondria into medical forebrain bundle (MFB) of 6-hydroxydopamine-unilaterally infused PD rats enhanced the survival of dopaminergic neurons and improved mitochondrial functions by recovering normal levels of mitochondrial complex I-IV and reducing mitochondrial oxidative stress in vivo [78].
Remarkably, the translational application of mitochondrial transfer should be further evaluated and its therapeutic potential exploited for the treatment of neurodegenerative diseases, such as AD and PD.
3.3. Mitochondrial Transfer in Neurodevelopmental Diseases: Extracellular Mitochondrial Release Reflecting Mitochondrial Dysfunction in Down Syndrome and Fragile X Syndrome
Mitochondrial dysfunction is a critical player contributing to the pathogenesis of several neurodevelopmental diseases, including Down syndrome (DS), the most common genetic defect leading to intellectual disability and caused by the trisomy of human chromosome 21. DS is characterized by neuropathological changes occurring already in fetal and neonatal life, leading to alterations in brain development [79].
Defective mitochondrial bioenergetics negatively compromise neuronal development and represent an early event in developing the neurobiological alterations characterizing the syndrome [80,81,82][80][81][82]. Although DS is a multi-genic disorder and many pathways are affected, oxidative phosphorylation (OXPHOS) dysfunction was found ubiquitously present in any tissue or cell type, regardless of age, including fetal one, so that DS is now regarded as an OXPHOS disorder [83].
In a very recent study, a new population of extracellular vesicles containing mitochondrial proteins, named “mitovesicles”, were identified and found altered in Down syndrome [84]. D’Acunzo et al. have shown that brain-derived mitochondria contain a specific subset of mitochondrial components and that their levels and cargo are aberrant in DS [84]. Comparative analysis of EVs derived from brains of Ts2 mouse model of DS and obtained from post-mortem human brains of individuals with DS showed higher numbers of mitovesicles with altered composition in the DS brain parenchyma, in both murine and human post-mortem brains [84] (Figure 2A). These data indicate that mitochondrial damage directly affects mitochondrial biology, either by activating the release of these vesicles or by regulating the mitochondrial cargo in the single extracellular vesicle.
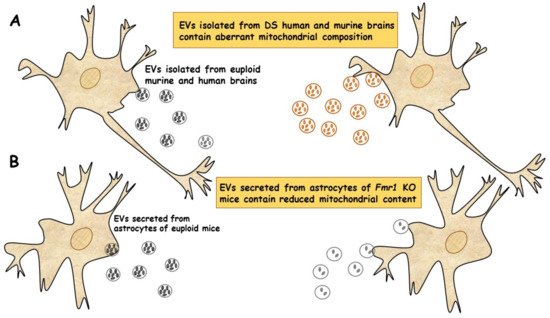
Figure 2. EVs of mitochondrial origin reflect mitochondrial dysfunction in DS and FXS. (A) Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in DS. Comparative analysis of EVs obtained from individuals with DS and Ts2 mouse model of DS showed higher numbers of mitovesicles with altered composition in the cerebral parenchyma, in both DS murine and human post-mortem brains [84]. (B) Depletion of mitochondrial components from extracellular vesicles secreted from astrocytes in a mouse model of FXS. Mitochondrial components contained in EVs derived from both cerebral cortices and astrocytes of Fmr1 KO mice were found reduced [85].
Taken together, these data show that mitochondrial levels and composition of mitovesicles mirror mitochondrial alterations within the cell of origin and could be used as biomarkers for assessing mitochondrial brain dysfunctions in neurological disorders.
Mitochondrial dysfunction contributes to the pathogenesis of another neurodevelopmental disease, the Fragile X syndrome (FXS). FXS is an inherited disorder characterized by mental retardation, caused by silencing of the fmr1 gene, encoding the Fragile X mental retardation protein (FMRP) [86], an RNA-binding protein expressed mainly in neurons and astrocytes of the brain and associated with approximately 4% of transcripts, including those for mitochondrial proteins [87]. Neuronal development in Fmr1 knock-out (KO) mice exhibited impaired dendritic maturation, altered expression of mitochondrial genes, fragmented mitochondria, impaired mitochondrial function and increased oxidative stress [88].
D’Antoni et al. provided the first evidence of a compromised and inefficient mitochondrial bioenergetics in the brain cortex of Fmr1 KO mice, a model of FXS, supporting the idea that mitochondrial dysfunctions may play a critical role in pathogenesis of the syndrome [89].
In a recent study, the ability of EVs to transfer mitochondrial components and their role in mitochondrial dysfunction was assessed in astrocytes and brain cortices from Fmr1 KO mice FLX model [85]. The mitochondrial protein levels of the transcription factor NRF-1 (nuclear respirator factor 1), the subunits ATP5A and ATPB of ATP synthase and the mitochondrial membrane protein VDAC1 in EVs were found drastically reduced in cerebral cortex and astrocyte samples from Fmr1 KO mice compared to euploid mice. These reductions are related to a reduction in mitochondrial biogenesis in the Fmr1 KO brain, associated with decreased mitochondrial membrane potential in Fmr1 KO astrocytes. Mitochondrial components were found reduced in both EVs derived from cerebral cortices and those secreted from astrocytes of Fmr1 KO mice (Figure 2B). The depletion of mitochondrial proteins contributes to mitochondrial dysfunction in astrocytes [85]. This study indicates that mitochondrial dysfunction in astrocytes is related to the pathogenesis of FXS and can be monitored by depletion of EV mitochondrial components.
These findings may improve the ability to diagnose neurodevelopmental diseases associated with mitochondrial dysfunction. However, this kind of study is nascent and further investigations are needed to define the exact mechanisms responsible for the observed decrease in mitochondrial proteins, which could mirror a deficit in intracellular transferring from mitochondria to EVs or a compromised EVs formation.
Furthermore, to better understand the complex physiological functions of astrocyte-derived EVs, it will be crucial to determine which components of EVs critically impact the progression of FXS disease and its regulatory mechanism.
References
- Bahat, A.; Gross, A. Mitochondrial plasticity in cell fate regulation. J. Biol. Chem. 2019, 294, 13852–13863.
- Lisowski, P.; Kannan, P.; Mlody, B.; Prigione, A. Mitochondria and the dynamic control of stem cell homeostasis. EMBO Rep. 2018, 19, e45432.
- Wallace, D.C. A Mitochondrial Paradigm of Metabolic and Degenerative Diseases, Aging, and Cancer: A Dawn for Evolutionary Medicine. Annu. Rev. Genet. 2005, 39, 359–407.
- Niyazov, D.M.; Kahler, S.G.; Frye, R.E. Primary Mitochondrial Disease and Secondary Mitochondrial Dysfunction: Importance of Distinction for Diagnosis and Treatment. Mol. Syndr. 2016, 7, 122–137.
- Mishra, P.; Chan, D.C. Metabolic regulation of mitochondrial dynamics. J. Cell Biol. 2016, 212, 379–387.
- Burté, F.; Carelli, V.; Chinnery, P.F.; Yu-Wai-Man, P. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat. Rev. Neurol. 2014, 11, 11–24.
- Glancy, B.; Hartnell, L.M.; Combs, C.A.; Femnou, A.; Sun, J.; Murphy, E.; Subramaniam, S.; Balaban, R.S. Power Grid Protection of the Muscle Mitochondrial Reticulum. Cell Rep. 2017, 19, 487–496.
- Mandal, A.; Drerup, C.M. Axonal Transport and Mitochondrial Function in Neurons. Front. Cell. Neurosci. 2019, 13, 373.
- Cunniff, B.; McKenzie, A.J.; Heintz, N.H.; Howe, A.K. AMPK activity regulates trafficking of mitochondria to the leading edge during cell migration and matrix invasion. Mol. Biol. Cell 2016, 27, 2662–2674.
- Schuler, M.-H.; Lewandowska, A.; Di Caprio, G.; Skillern, W.; Upadhyayula, S.; Kirchhausen, T.; Shaw, J.M.; Cunniff, B. Miro1-mediated mitochondrial positioning shapes intracellular energy gradients required for cell migration. Mol. Biol. Cell 2017, 28, 2159–2169.
- Torralba, D.; Baixauli, F.; Sánchez-Madrid, F. Mitochondria Know No Boundaries: Mechanisms and Functions of Intercellular Mitochondrial Transfer. Front. Cell Dev. Biol. 2016, 4, 107.
- Singh, B.; Modica-Napolitano, J.S.; Singh, K.K. Defining the momiome: Promiscuous information transfer by mobile mitochondria and the mitochondrial genome. Semin. Cancer Biol. 2017, 47, 1–17.
- Sinha, P.; Islam, M.N.; Bhattacharya, S.; Bhattacharya, J. Intercellular mitochondrial transfer: Bioenergetic crosstalk between cells. Curr. Opin. Genet. Dev. 2016, 38, 97–101.
- Shanmughapriya, S.; Langford, D.; Natarajaseenivasan, K. Inter and Intracellular mitochondrial trafficking in health and disease. Ageing Res. Rev. 2020, 62, 101128.
- Liu, D.; Gao, Y.; Liu, J.; Huang, Y.; Yin, J.; Feng, Y.; Shi, L.; Meloni, B.P.; Zhang, C.; Zheng, M.; et al. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct. Target. Ther. 2021, 6, 1–18.
- Qin, Y.; Jiang, X.; Yang, Q.; Zhao, J.; Zhou, Q.; Zhou, Y. The Functions, Methods, and Mobility of Mitochondrial Transfer Between Cells. Front. Oncol. 2021, 11.
- Gerdes, H.-H.; Bukoreshtliev, N.V.; Barroso, J.F. Tunneling nanotubes: A new route for the exchange of components between animal cells. FEBS Lett. 2007, 581, 2194–2201.
- Dupont, M.; Souriant, S.; Lugo, G.; Maridonneau-Parini, I.; Vérollet, C. Tunneling Nanotubes: Intimate Communication between Myeloid Cells. Front. Immunol. 2018, 9, 43.
- Li, R.-F.; Zhang, W.; Man, Q.-W.; Zhao, Y.-F.; Zhao, Y. Tunneling nanotubes mediate intercellular communication between endothelial progenitor cells and osteoclast precursors. J. Mol. Histol. 2019, 50, 483–491.
- Panasiuk, M.; Rychłowski, M.; Derewońko, N.; Bieńkowska-Szewczyk, K. Tunneling Nanotubes as a Novel Route of Cell-to-Cell Spread of Herpesviruses. J. Virol. 2018, 92.
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.-H. Nanotubular Highways for Intercellular Organelle Transport. Science 2004, 303, 1007–1010.
- Bukoreshtliev, N.V.; Wang, X.; Hodneland, E.; Gurke, S.; Barroso, J.F.; Gerdes, H.-H. Selective block of tunneling nanotube (TNT) formation inhibits intercellular organelle transfer between PC12 cells. FEBS Lett. 2009, 583, 1481–1488.
- Ahmad, T.; Mukherjee, S.; Pattnaik, B.; Kumar, M.; Singh, S.; Rehman, R.; Tiwari, B.K.; Jha, K.A.; Barhanpurkar, A.P.; Wani, M.R.; et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014, 33, 994–1010.
- Wang, Y.; Cui, J.; Sun, X.; Zhang, Y. Tunneling-nanotube development in astrocytes depends on p53 activation. Cell Death Differ. 2010, 18, 732–742.
- Marlein, C.R.; Piddock, R.E.; Mistry, J.J.; Zaitseva, L.; Hellmich, C.; Horton, R.H.; Zhou, Z.; Auger, M.J.; Bowles, K.M.; Rushworth, S.A. CD38-Driven Mitochondrial Trafficking Promotes Bioenergetic Plasticity in Multiple Myeloma. Cancer Res. 2019, 79, 2285–2297.
- Hekmatshoar, Y.; Nakhle, J.; Galloni, M.; Vignais, M.L. The role of metabolism and tunneling nanotube-mediated intercellular mitochondria exchange in cancer drug resistance. Biochem. J. 2018, 475, 2305–2328.
- Li, C.; Cheung, M.K.H.; Han, S.; Zhang, Z.; Chen, L.; Chen, J.; Zeng, H.; Qiu, J. Mesenchymal stem cells and their mitochondrial transfer: A double-edged sword. Biosci. Rep. 2019, 39.
- Gousset, K.; Marzo, L.; Commere, P.-H.; Zurzolo, C. Myo10 is a key regulator of TNT formation in neuronal cells. J. Cell Sci. 2013, 126, 4424–4435.
- Sun, X.; Wang, Y.; Zhang, J.; Tu, J.; Wang, X.-J.; Su, X.-D.; Wang, L.; Zhang, Y. Tunneling-nanotube direction determination in neurons and astrocytes. Cell Death Dis. 2012, 3, e438.
- Ham, P.B.; Raju, R. Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog. Neurobiol. 2017, 157, 92–116.
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial transfer from bone-marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012, 18, 759–765.
- Plotnikov, E.Y.; Babenko, V.A.; Silachev, D.; Zorova, L.D.; Khryapenkova, T.G.; Savchenko, E.S.; Pevzner, I.B.; Zorov, D.B. Intercellular transfer of mitochondria. Biochemistry 2015, 80, 542–548.
- Paliwal, S.; Chaudhuri, R.; Agrawal, A.; Mohanty, S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J. Biomed. Sci. 2018, 25, 1–12.
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016, 7, 1–13.
- Zappulli, V.; Friis, K.P.; Fitzpatrick, Z.; Maguire, C.A.; Breakefield, X.O. Extracellular vesicles and intercellular communication within the nervous system. J. Clin. Investig. 2016, 126, 1198–1207.
- Pitt, J.M.; Kroemer, G.; Zitvogel, L. Extracellular vesicles: Masters of intercellular communication and potential clinical interventions. J. Clin. Investig. 2016, 126, 1139–1143.
- Paolicelli, R.C.; Bergamini, G.; Rajendran, L. Cell-to-cell Communication by Extracellular Vesicles: Focus on Microglia. Neuroscience 2019, 405, 148–157.
- Zaborowski, M.; Balaj, L.; Breakefield, X.O.; Lai, C.P.-K. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797.
- Simeone, P.; Bologna, G.; Lanuti, P.; Pierdomenico, L.; Guagnano, M.T.; Pieragostino, D.; Del Boccio, P.; Vergara, D.; Marchisio, M.; Miscia, S.; et al. Extracellular Vesicles as Signaling Mediators and Disease Biomarkers across Biological Barriers. Int. J. Mol. Sci. 2020, 21, 2514.
- Guescini, M.; Genedani, S.; Stocchi, V.; Agnati, L.F. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J. Neural Transm. 2009, 117, 1–4.
- Sansone, P.; Savini, C.; Kurelac, I.; Chang, Q.; Amato, L.B.; Strillacci, A.; Stepanova, A.; Iommarini, L.; Mastroleo, C.; Daly, L.; et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E9066–E9075.
- Phinney, D.; Di Giuseppe, M.; Njah, J.; Sala-Llinas, E.; Shiva, S.; Croix, C.M.S.; Stolz, D.B.; Watkins, S.; Di, Y.P.; Leikauf, G.; et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 2015, 6, 8472.
- Davis, C.-H.O.; Kim, K.-Y.; Bushong, E.A.; Mills, E.A.; Boassa, D.; Shih, T.; Kinebuchi, M.; Phan, S.; Zhou, Y.; Bihlmeyer, N.; et al. Transcellular degradation of axonal mitochondria. Proc. Natl. Acad. Sci. USA 2014, 111, 9633–9638.
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Ji, X.; Lo, E.H. Transfer of mitochondria from astrocytes to neurons after stroke. Nat. Cell Biol. 2016, 535, 551–555.
- Mistry, J.J.; Marlein, C.R.; Moore, J.A.; Hellmich, C.; Wojtowicz, E.E.; Smith, J.; Macaulay, I.; Sun, Y.; Morfakis, A.; Patterson, A.; et al. ROS-mediated PI3K activation drives mitochondrial transfer from stromal cells to hematopoietic stem cells in response to infection. Proc. Natl. Acad. Sci. USA 2019, 116, 24610–24619.
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.; O’Kane, C.; Krasnodembskaya, A.D. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286.
- Otsu, K.; Das, S.; Houser, S.D.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood 2009, 113, 4197–4205.
- Yao, Y.; Fan, X.-L.; Jiang, D.; Zhang, Y.; Li, X.; Xu, Z.-B.; Fang, S.-B.; Chiu, S.; Tse, H.-F.; Lian, Q.; et al. Connexin 43-mediated mitochondrial transfer of iPSC-MSCs alleviates asthma inflammation. Stem Cell Rep. 2018, 11, 1120–1135.
- Ishikawa, E.T.; Nieto, D.G.; Ghiaur, G.; Dunn, S.K.; Ficker, A.M.; Murali, B.; Madhu, M.; Gutstein, D.E.; Fishman, G.I.; Barrio, L.C.; et al. Connexin-43 prevents hematopoietic stem cell senescence through transfer of reactive oxygen species to bone marrow stromal cells. Proc. Natl. Acad. Sci. USA 2012, 109, 9071–9076.
- Lyamzaev, K.G.; Nepryakhina, O.K.; Saprunova, V.B.; Bakeeva, L.E.; Pletjushkina, O.Y.; Chernyak, B.; Skulachev, V.P. Novel mechanism of elimination of malfunctioning mitochondria (mitoptosis): Formation of mitoptotic bodies and extrusion of mitochondrial material from the cell. Biochim. Biophys. Acta (BBA) Bioenerg. 2008, 1777, 817–825.
- Nakajima, A.; Kurihara, H.; Yagita, H.; Okumura, K.; Nakano, H. Mitochondrial Extrusion through the Cytoplasmic Vacuoles during Cell Death. J. Biol. Chem. 2008, 283, 24128–24135.
- Boudreau, L.H.; Duchez, A.-C.; Cloutier, N.; Soulet, D.; Martin, N.; Bollinger, J.; Paré, A.; Rousseau, M.; Naika, G.S.; Lévesque, T.; et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 2014, 124, 2173–2183.
- Aguilar, P.; Baylies, M.K.; Fleissner, A.; Helming, L.; Inoue, N.; Podbilewicz, B.; Wang, H.; Wong, M. Genetic basis of cell–cell fusion mechanisms. Trends Genet. 2013, 29, 427–437.
- Huang, P.-J.; Kuo, C.-C.; Lee, H.-C.; Shen, C.-I.; Cheng, F.-C.; Wu, S.-F.; Chang, J.C.; Pan, H.-C.; Lin, S.-Z.; Liu, C.-S.; et al. Transferring Xenogenic Mitochondria Provides Neural Protection against Ischemic Stress in Ischemic Rat Brains. Cell Transplant. 2016, 25, 913–927.
- Lippert, T.; Borlongan, C.V. Prophylactic treatment of hyperbaric oxygen treatment mitigates inflammatory response via mitochondria transfer. CNS Neurosci. Ther. 2019, 25, 815–823.
- Wang, X.F.; Cynader, M.S. Pyruvate Released by Astrocytes Protects Neurons from Copper-Catalyzed Cysteine Neurotoxicity. J. Neurosci. 2001, 21, 3322–3331.
- Ouyang, Y.-B.; Xu, L.; Lu, Y.; Sun, X.; Yue, S.; Xiong, X.; Giffard, R.G. Astrocyte-enriched miR-29a targets PUMA and reduces neuronal vulnerability to forebrain ischemia. Glia 2013, 61, 1784–1794.
- Joshi, A.U.; Minhas, P.S.; Liddelow, S.A.; Haileselassie, B.; Andreasson, K.I.; Ii, G.W.D.; Mochly-Rosen, D. Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nat. Neurosci. 2019, 22, 1635–1648.
- Cheng, X.-Y.; Biswas, S.; Li, J.; Mao, C.-J.; Chechneva, O.; Chen, J.; Li, K.; Li, J.; Zhang, J.-R.; Liu, C.-F.; et al. Human iPSCs derived astrocytes rescue rotenone-induced mitochondrial dysfunction and dopaminergic neurodegeneration in vitro by donating functional mitochondria. Transl. Neurodegener. 2020, 9, 1–14.
- English, K.; Shepherd, A.; Uzor, N.-E.; Trinh, R.; Kavelaars, A.; Heijnen, C.J. Astrocytes rescue neuronal health after cisplatin treatment through mitochondrial transfer. Acta Neuropathol. Commun. 2020, 8, 1–14.
- Hayakawa, K.; Bruzzese, M.; Chou, S.H.-Y.; Ning, M.; Ji, X.; Lo, E.H. Extracellular Mitochondria for Therapy and Diagnosis in Acute Central Nervous System Injury. JAMA Neurol. 2018, 75, 119–122.
- Hayakawa, K.; Chan, S.J.; Mandeville, E.T.; Park, J.H.; Bruzzese, M.; Montaner, J.; Arai, K.; Rosell, A.; Lo, E.H. Protective Effects of Endothelial Progenitor Cell-Derived Extracellular Mitochondria in Brain Endothelium. Stem Cells 2018, 36, 1404–1410.
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020, 15, 1–22.
- Park, J.-S.; Davis, R.; Sue, C.M. Mitochondrial Dysfunction in Parkinson’s Disease: New Mechanistic Insights and Therapeutic Perspectives. Curr. Neurol. Neurosci. Rep. 2018, 18, 1–11.
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615.
- Swerdlow, R.H. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1403–1416.
- Nitzan, K.; Benhamron, S.; Valitsky, M.; Kesner, E.E.; Lichtenstein, M.; Ben-Zvi, A.; Ella, E.; Segalstein, Y.; Saada, A.; Lorberboum-Galski, H.; et al. Mitochondrial Transfer Ameliorates Cognitive Deficits, Neuronal Loss, and Gliosis in Alzheimer’s Disease Mice. J. Alzheimer’s Dis. 2019, 72, 587–604.
- Marino, B.L.; De Souza, L.R.; Sousa, K.P.; Ferreira, J.V.; Padilha, E.C.; Da Silva, C.H.; Taft, C.A.; Hage-Melim, L.I. Parkinson’s Disease: A Review from Pathophysiology to Treatment. Mini-Rev. Med. Chem. 2020, 20, 754–767.
- McCann, H.; Stevens, C.H.; Cartwright, H.; Halliday, G.M. α-Synucleinopathy phenotypes. Parkinsonism Relat. Disord. 2014, 20, S62–S67.
- Valdinocci, D.; Radford, R.A.W.; Siow, S.M.; Chung, R.S.; Pountney, D.L. Potential Modes of Intercellular α-Synuclein Transmission. Int. J. Mol. Sci. 2017, 18, 469.
- Bantle, C.M.; Hirst, W.D.; Weihofen, A.; Shlevkov, E. Mitochondrial Dysfunction in Astrocytes: A Role in Parkinson’s Disease? Front. Cell Dev. Biol. 2021, 8.
- Chen, C.; Turnbull, D.M.; Reeve, A.K. Mitochondrial dysfunction in Parkinson’s disease—cause or consequence? Biology 2019, 8, 38.
- Celardo, I.; Martins, L.M.; Gandhi, S. Unravelling mitochondrial pathways to Parkinson’s disease. Br. J. Pharmacol. 2014, 171, 1943–1957.
- Valdinocci, D.; Simões, R.; Kovarova, J.; Cunha-Oliveira, T.; Neuzil, J.; Pountney, D.L. Intracellular and Intercellular Mitochondrial Dynamics in Parkinson’s Disease. Front. Neurosci. 2019, 13, 930.
- Morales, I.; Sanchez, A.; Puertas-Avendaño, R.; Rodriguez-Sabate, C.; Perez-Barreto, A.; Rodriguez, M. Neuroglial transmitophagy and Parkinson’s disease. Glia 2020.
- Barodia, S.K.; McMeekin, L.J.; Creed, R.B.; Quinones, E.K.; Cowell, R.M.; Goldberg, M.S. PINK1 phosphorylates ubiquitin predominantly in astrocytes. NPJ Park. Dis. 2019, 5, 1–9.
- Chang, J.C.; Wu, S.-L.; Liu, K.-H.; Chen, Y.-H.; Chuang, C.-S.; Cheng, F.-C.; Su, H.-L.; Wei, Y.-H.; Kuo, S.-J.; Liu, C.-S. Allogeneic/xenogeneic transplantation of peptide-labeled mitochondria in Parkinson’s disease: Restoration of mitochondria functions and attenuation of 6-hydroxydopamine–induced neurotoxicity. Transl. Res. 2016, 170, 40–56.e3.
- Kazemi, M.; Salehi, M.; Kheirollahi, M. Down syndrome: Current status, challenges and future perspectives. Int. J. Mol. Cell Med. 2016, 5, 125–133.
- Valenti, D.; de Bari, L.; De Filippis, B.; Henrion-Caude, A.; Vacca, R.A. Mitochondrial dysfunction as a central actor in intellectual disability-related diseases: An overview of Down syndrome, autism, Fragile X and Rett syndrome. Neurosci. Biobehav. Rev. 2014, 46, 202–217.
- Valenti, D.; Braidy, N.; De Rasmo, D.; Signorile, A.; Rossi, L.; Atanasov, A.; Volpicella, M.; Henrion-Caude, A.; Nabavi, S.; Vacca, R. Mitochondria as pharmacological targets in Down syndrome. Free Radic. Biol. Med. 2018, 114, 69–83.
- Vacca, R.A.; Bawari, S.; Valenti, D.; Tewari, D.; Nabavi, S.F.; Shirooie, S.; Sah, A.N.; Volpicella, M.; Braidy, N. Down syndrome: Neurobiological alterations and therapeutic targets. Neurosci. Biobehav. Rev. 2019, 98, 234–255.
- Bayona-Bafaluy, M.P.; Garrido-Pérez, N.; Meade, P.; Iglesias, E.; Jiménez-Salvador, I.; Montoya, J.; Martínez-Cué, C.; Ruiz-Pesini, E. Down syndrome is an oxidative phosphorylation disorder. Redox Biol. 2021, 41, 101871.
- D’Acunzo, P.; Pérez-González, R.; Kim, Y.; Hargash, T.; Miller, C.; Alldred, M.J.; Erdjument-Bromage, H.; Penikalapati, S.C.; Pawlik, M.; Saito, M.; et al. Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in Down syndrome. Sci. Adv. 2021, 7, eabe5085.
- Saldarriaga, W.; Tassone, F.; González-Teshima, L.Y.; Forero-Forero, J.V.; Ayala-Zapata, S.; Hagerman, R. Fragile X syndrome. Colomb. Med. 2014, 45, 190–198.
- Potter, M.; Newport, E.; Morten, K.J. The Warburg effect: 80 years on. Biochem. Soc. Trans. 2016, 44, 1499–1505.
- Brown, V.; Jin, P.; Ceman, S.; Darnell, J.C.; O’Donnell, W.T.; Tenenbaum, S.A.; Jin, X.; Feng, Y.; Wilkinson, K.D.; Keene, J.D.; et al. Microarray Identification of FMRP-Associated Brain mRNAs and Altered mRNA Translational Profiles in Fragile X Syndrome. Cell 2001, 107, 477–487.
- Shen, M.; Wang, F.; Li, M.; Sah, N.; Stockton, M.; Tidei, J.J.; Gao, Y.; Korabelnikov, T.; Kannan, S.; Vevea, J.D.; et al. Reduced mitochondrial fusion and Huntingtin levels contribute to impaired dendritic maturation and behavioral deficits in Fmr1-mutant mice. Nat. Neurosci. 2019, 22, 386–400.
- D’Antoni, S.; De Bari, L.; Valenti, D.; Borro, M.; Bonaccorso, C.M.; Simmaco, M.; Vacca, R.A.; Catania, M.V. Aberrant mitochondrial bioenergetics in the cerebral cortex of the Fmr1 knockout mouse model of fragile X syndrome. Biol. Chem. 2019, 401, 497–503.
- Ha, B.G.; Heo, J.-Y.; Jang, Y.-J.; Park, T.-S.; Choi, J.-Y.; Jang, W.Y.; Jeong, S.-J. Depletion of Mitochondrial Components from Extracellular Vesicles Secreted from Astrocytes in a Mouse Model of Fragile X Syndrome. Int. J. Mol. Sci. 2021, 22, 410.
More
