Amperometric biosensors utilizing oxidoreductases were classified into three generations: 1st generation biosensors employing oxidases based on the electrocatalytic monitoring of substrate consumption or product formation, 2nd generation biosensors employing oxidases or dehydrogenases based on the electrocatalytic recycling of suitable redox mediators, and 3rd generation biosensors employing oxidoreductases capable of direct electron transfer to bare or modified electrodes.
- biosensor
- current density
- direct electron transfer
- enzyme
- selectivity
- sensitivity
- nanomaterial
- self-assembled monolayers
- nanocomposite
- nanoparticle
1. Introduction
Chemical sensors consist of a chemical recognition system, a physicochemical transducer and a signal processing unit that transforms the concentration of the analyte into a quantifiable signal. A biosensor employs a biological recognition (biorecognition) element working on the principle of bioaffinity or biocatalysis. Bioaffinity sensors use receptor proteins, antibodies, or polynucleotides as biorecognition elements, whereas enzymes are used as bioelectrocatalysts. Several types of signal transducers can be distinguished, for example: mass-sensitive, optical, and electrochemical. Electrochemical biosensors can be based on the detection of a measurable current (amperometric), the accumulation of charge (potentiometric), changes in current as a result of a potentiometric effect at a gate electrode (field effect), changes in the conductive properties of a medium between electrodes (conductometric), or the changes of resistance and reactance close to an electrode (impedimetric).
Amperometric biosensors utilizing oxidoreductases were classified into three generations (Figure 1) as indicated above. Direct electron transfer (DET) forms the basis of 3rd generation biosensors [1]. DET is the ability of a solubilized or immobilized redox active protein to exchange electrons with an electrode upon contact; for example, oxidoreductases directly donating electrons gained from substrate oxidation to the electrode. Therefore, 3rd generation biosensors can be operated at lower potentials (close to the midpoint potential of the enzyme’s prosthetic group) than those needed for the detection of enzymatic reaction products, e.g., hydrogen peroxide, in 1st generation biosensors, or even the redox potential needed to apply redox mediators to shuttle electrons between the enzyme cofactor and the electrode in 2nd generation biosensors. The specificity of 3rd generation biosensors is thus less affected by electroactive interferents like ascorbic acid and also by the absence of the redox active mediator.
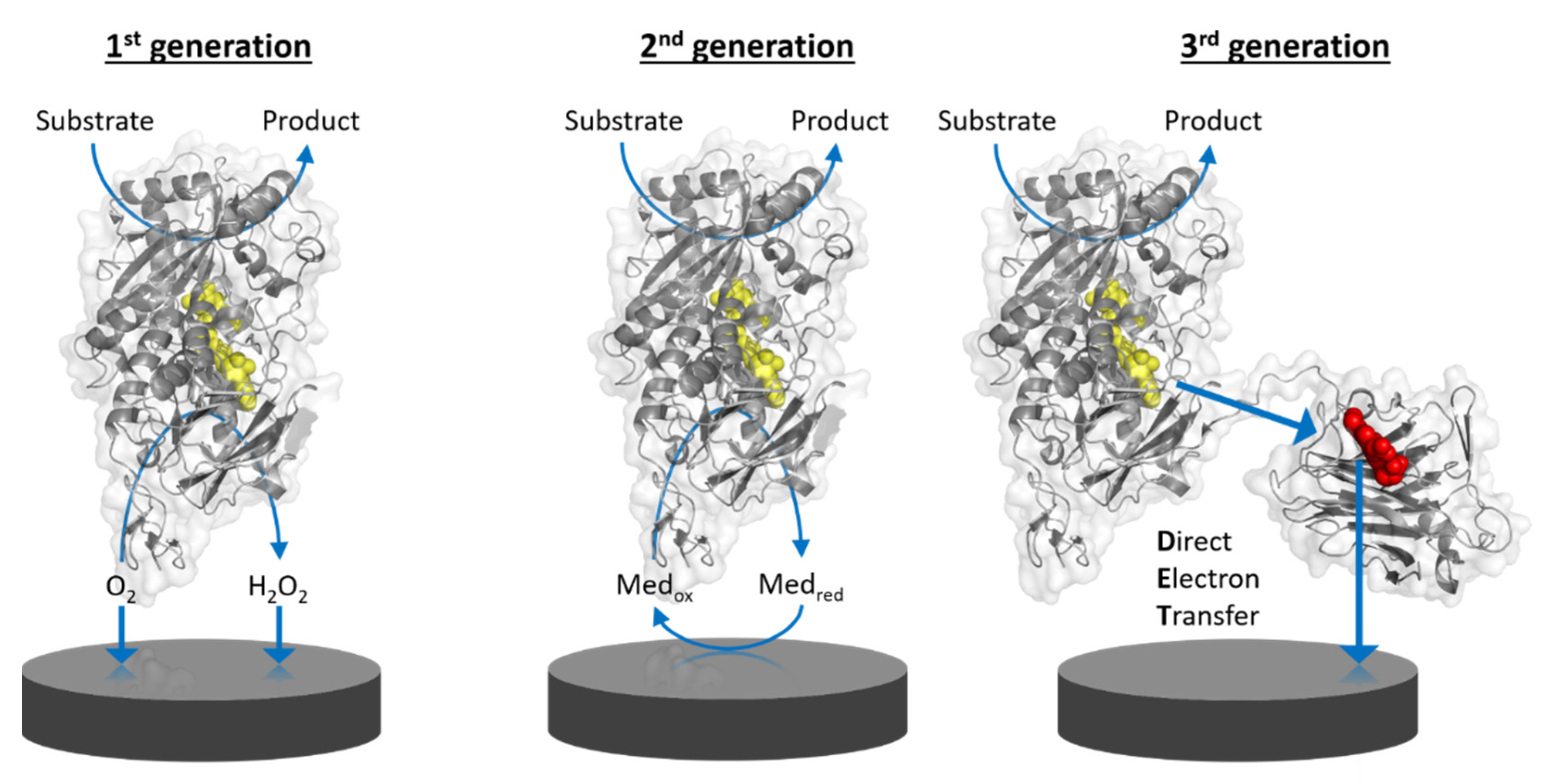 Figure 1. Schematic representation of the working principle of the three generations of enzyme-based amperometric biosensors. 1st generation: based on the electrocatalytic detection of enzymatic substrate conversion or product formation. 2nd generation: based on the electrocatalytic recycling of a redox mediator. 3rd generation: based on direct electron transfer from enzyme to electrode. Yellow molecules depict the FAD cofactor of flavoenzymes and the red molecule depicts the heme cofactor in the mobile cytochrome domain of a multi-cofactor enzyme.
Figure 1. Schematic representation of the working principle of the three generations of enzyme-based amperometric biosensors. 1st generation: based on the electrocatalytic detection of enzymatic substrate conversion or product formation. 2nd generation: based on the electrocatalytic recycling of a redox mediator. 3rd generation: based on direct electron transfer from enzyme to electrode. Yellow molecules depict the FAD cofactor of flavoenzymes and the red molecule depicts the heme cofactor in the mobile cytochrome domain of a multi-cofactor enzyme.
2. Applications and Challenges of DET-Based Biosensors
2.1. Applications for DET-Based Biosensors
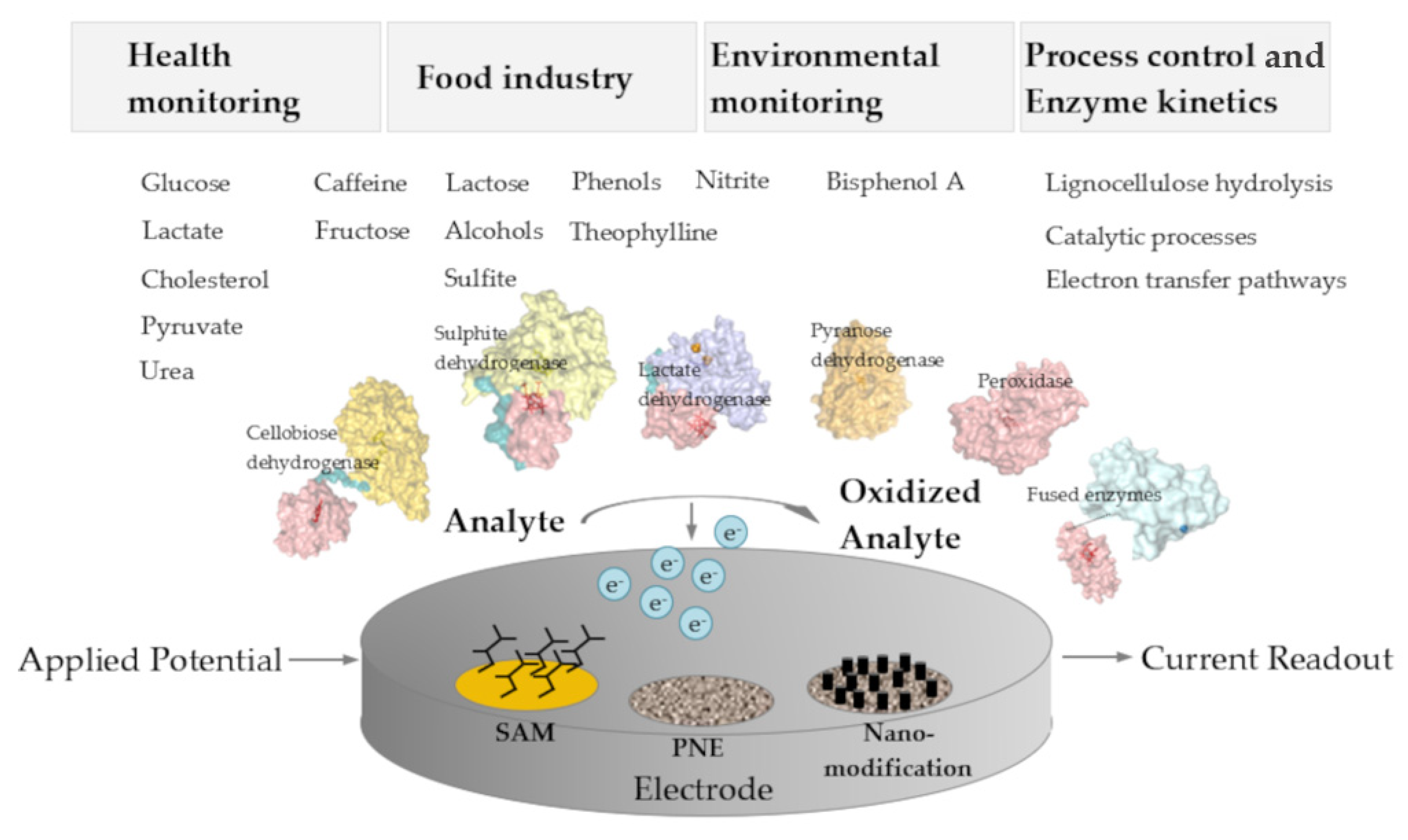 Figure 2. Schematic overview on application areas, analytes, enzymes, and the architecture of 3rd generation amperometric biosensors. SAM, self-assembled monolayer; PNE, porous nanostructured electrodes.
Figure 2. Schematic overview on application areas, analytes, enzymes, and the architecture of 3rd generation amperometric biosensors. SAM, self-assembled monolayer; PNE, porous nanostructured electrodes.2.2. Challenges of DET-Based Biosensors
3. Conclusions and Future Perspectives
Direct electrochemistry of oxidoreductases is utilized to generate biosensors for simple and low-priced on-line quantification of analytes. The analyte spectrum covers a broad range of relevant analytes like saccharides, phenolic compounds, and small molecules such as sulfite or alcohols, which are relevant for environmental monitoring, compounds of the food industry, and products of biotechnological processes. Many reports in the literature focus on glucose detection because of the high medical relevance for blood glucose determination, although much more enzymes with suitable electrochemical properties exist and can be applied to detect other substrates. Several of those are capable of DET and can be used for 3rd generation sensors when combined with the correct combination of electrode materials and various electrode modifications. The performance of DET-based biosensors has to be carefully reported and the necessary control experiments to rule out mediated electron transfer should be provided. Despite this point of caution, many studies on DET enzymes and 3rd generation biosensors are performed with great care and show a high level of insight and expertise. Some research groups particularly focus on the optimized electrode material design employing nanomaterials and investigate optimized fabrication and protein immobilization strategies to achieve biosensors with higher sensitivity and stability. To date only one commercially available DET-based amperometric 3rd generation biosensor (LactoSens) exists compared to a great number of existing 1st and 2nd generation biosensors. Since not all oxidoreductases are capable of DET, the fusion of enzymes with electron-transferring cytochromes is a very promising strategy to increase the number of bioelectrocatalysts for 3rd generation biosensors and to increase the number of analytes.
References
- Gorton, L.; Lindgren, A.; Larsson, T.; Munteanu, F.D.; Ruzgas, T.; Gazaryan, I. Direct Electron Transfer between Heme-Containing Enzymes and Electrodes as Basis for Third Generation Biosensors. Anal. Chim. Acta 1999, 400, 91–108.
- Cruys-Bagger, N.; Ren, G.; Tatsumi, H.; Baumann, M.J.; Spodsberg, N.; Andersen, H.D.; Gorton, L.; Borch, K.; Westh, P. An Amperometric Enzyme Biosensor for Real-Time Measurements of Cellobiohydrolase Activity on Insoluble Cellulose. Biotechnol. Bioeng. 2012, 109, 3199–3204.
- Chang, H.; Wohlschlager, L.; Csarman, F.; Ruff, A.; Schuhmann, W.; Scheiblbrandner, S.; Ludwig, R. Real-Time Measurement of Cellobiose and Glucose Formation during Enzymatic Biomass Hydrolysis. Anal. Chem. 2021, 93, 7732–7738.
- Gray, H.B.; Winkler, J.R. Electron Tunneling through Proteins. Q. Rev. Biophys. 2003, 36, 341–372.
- Moser, C.C.; Keske, J.M.; Warncke, K.; Farid, R.S.; Dutton, P.L. Nature of Biological Electron Transfer. Nature 1992, 355, 796–802.
- Marcus, R.A.; Sutin, N. Electron Transfers in Chemistry and Biology. Biochim. Biophys. Acta 1985, 811, 265–322.
- Mayo, S.L.; Ellis, W.R.; Crutchley, R.J.; Gray, H.B. Long-Range Electron Transfer in Heme Proteins. Science 1986, 233, 953–959.
- Gulaboski, R.; Mirčeski, V.; Kappl, R.; Hoth, M.; Bozem, M. Review—Quantification of Hydrogen Peroxide by Electrochemical Methods and Electron Spin Resonance Spectroscopy. J. Electrochem. Soc. 2019, 166, G82–G101.
- Chenault, H.K.; Whitesides, G.M. Regeneration of Nicotinamide Cofactors for Use in Organic Synthesis. Appl. Biochem. Biotechnol. 1987, 14, 147–197.
- Clark, W.M. Oxidation–Reduction Potentials of Organic Systems. J. Chem. Educ. 1960, 38, 487.
- Saleh, F.S.; Rahman, M.R.; Okajima, T.; Mao, L.; Ohsaka, T. Determination of Formal Potential of NADH/NAD+ Redox Couple and Catalytic Oxidation of NADH Using Poly(Phenosafranin)-Modified Carbon Electrodes. Bioelectrochemistry 2011, 80, 121–127.
- Emahi, I.; Mitchell, M.P.; Baum, D.A. Electrochemistry of Pyrroloquinoline Quinone (PQQ) on Multi-Walled Carbon Nanotube-Modified Glassy Carbon Electrodes in Biological Buffers. J. Electrochem. Soc. 2017, 164, H3097–H3102.
- Luong, J.H.T.; Glennon, J.D.; Gedanken, A.; Vashist, S.K. Achievement and Assessment of Direct Electron Transfer of Glucose Oxidase in Electrochemical Biosensing Using Carbon Nanotubes, Graphene, and Their Nanocomposites. Microchim. Acta 2017, 184, 369–388.
- Liu, Y.; Wang, M.; Zhao, F.; Xu, Z.; Dong, S. The Direct Electron Transfer of Glucose Oxidase and Glucose Biosensor Based on Carbon Nanotubes/Chitosan Matrix. Biosens. Bioelectron. 2005, 21, 984–988.
- Suzuki, N.; Lee, J.; Loew, N.; Takahashi-Inose, Y.; Okuda-Shimazaki, J.; Kojima, K.; Mori, K.; Tsugawa, W.; Sode, K. Engineered Glucose Oxidase Capable of Quasi-Direct Electron Transfer after a Quick-and-Easy Modification with a Mediator. Int. J. Mol. Sci. 2020, 21, 1137.
- Yamazaki, T.; Okuda-Shimazaki, J.; Sakata, C.; Tsuya, T.; Sode, K. Construction and Characterization of Direct Electron Transfer-Type Continuous Glucose Monitoring System Employing Thermostable Glucose Dehydrogenase Complex. Anal. Lett. 2008, 41, 2363–2373.
