Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Udo Jeschke and Version 2 by Rita Xu.
Trace amine-associated receptor 1 (TAAR1) is a Gαs- protein coupled receptor that plays an important role in the regulation of the immune system and neurotransmission in the CNS. In ovarian cancer cell lines, stimulation of TAAR1 via 3-iodothyronamine (T1AM) reduces cell viability and induces cell death and DNA damage.
- TAAR1
- ovarian cancer
- prognostic factor
- immunohistochemistry
1. Introduction
With more than 7000 new cases in Germany in 2016, ovarian cancer (OC) is representing 3.1% of cancer cases and 5.2% of cancer deaths in females [1]. Due to its high potential for cancer dissemination into the peritoneum, frequent late symptoms and detection, heterogeneity, and acquired and intrinsic chemoresistance [2][3][2,3], epithelial ovarian cancer shows relative 5 years survival rates of less than 50% [4] with only slight improvement and a wide range of 5 years survival rates in geographic distribution [5]. OC presents in a histologically diverse way, with epithelial type being the most frequent, accounting for more than 90% of all primary ovarian tumors [6]. Other histological subtypes include sex cord-stromal (5–6%), germ cell (2–3%), and rare miscellaneous entities [6]. This article focuses on epithelial tumors, which can be subdivided into high-grade serous carcinomas (70–80% of cases), low-grade serous carcinomas, endometrioid (<5%), clear cell (3%), and mucinous cancers (<3%) [7].
Therapeutic management of OC lacks effective screening methods. Clinical prognosticators such as disease stage at diagnosis, postsurgical residual disease, histological subtype, grading, mutation in the breast cancer gene (BRCA), general state of health, age, guideline-based therapy, and volume of ascites are of value [1][3][1,3]. Platinum resistance after chemotherapy can worsen the prognosis of ovarian cancer patients [8][9][8,9]. Attempts of establishing new prognostic factors have been published in multiple studies [10][11][12][10,11,12].
Ovarian cancer is regulated by thyroid hormones and its derivatives [13][14][13,14]. Thyroid hormones also have a pro-angiogenic role in various cancer types, making its receptors potential targets for therapeutic treatment [15]. Similar to ovarian cancer, hypothyroidism occurs predominantly in aging women [16][17][16,17]. Hyperthyroidism is prevalent in women from the 3rd to 5th decade of life with an 8:1 female:male ratio [18].
Thyronamines, such as 3-iodothyronamine (T1AM), derive from thyroid hormones [19] via degradation by the enzyme ornithine decarboxylase (ODC) and intestinal deiodinases [20]. In comparison with the mostly epigenetically acting classical thyroid hormones, decarboxylated thyroid hormones can function rapidly (e.g., via fast change of heart rate and body temperature) [19]. T1AM is described to inhibit cell growth and viability, induce cell death, and lead to DNA damage in ovarian cancer cells [13]. Furthermore, T1AM and its metabolites, T0AM and thyroacetic acid (TA1), can influence pancreatic islets, brain, heart, and other tissues through the Gαs-protein coupled receptor trace amine-associated receptor 1 (TAAR1) [20]. Other agonists of TAAR1 are trace amines, which are closely associated metabolically with the dopamine, serotonin, and noradrenaline systems [21]. The degradation and synthesis of both trace amines and thyronamines proceed via enzymes working through decarboxylation. L-thyroxin (T4) is degraded to thyronamines (T1AM) via ornithine decarboxylase (ODC) [20] while trace amines are synthesized via L-amino acid decarboxylase [21]. TAAR1 is widely expressed including placenta, brain, spinal cord, immune cells such as leukocytes, macrophages and dendritic cells, breast cancer tissue, D-cells in stomach, and pancreatic β cells [22][23][24][25][26][22,23,24,25,26]. Activation of TAAR1 leads to a GαS-protein mediated increase in intracellular cAMP levels [27][28][27,28].
2. Differences of TAAR1 Expression in Histological Subtypes of Ovarian Cancer
TAAR1 was detected in membrane as well as in cytoplasm. Membrane TAAR1 staining could be gained in n = 134 cases and presented in endometrioid tumors (n = 16) and serous carcinoma (n = 98), with a median IRS of 3, in clear cell (n = 9) and mucinous tumor (n = 11) with a median IRS of 1 (p = 0.003), as shown in Figure 1. In n = 22 cases, either staining was not successful, or no ovarian cancer tissue was hit.
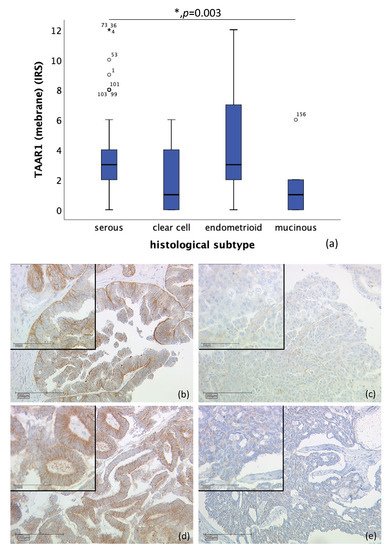

Figure 1. Correlation of membrane TAAR1 expression with histological subtype (p = 0.003) (a) boxplot of membrane TAAR1 expression and histological subtype (b) serous carcinoma (n = 98) with a membrane TAAR1 IRS of 4, magnification ×10 and ×25 in the inset (c) clear cell carcinoma (n = 9) with a membrane TAAR1 IRS of 1, magnification ×10 and ×25 in the inset (d) endometrioid carcinoma (n = 16) with a membrane TAAR1 IRS of 4, magnification ×10 and ×25 in the inset (e) mucinous carcinoma (n = 11) with a membrane TAAR1 IRS of 0, magnification ×10 and ×25 in the inset; * p < 0.05 was considered statistically significant.
Likewise, correlation of cytoplasmic TAAR1 expression and histological subtype differs significantly. A total of n = 128 cases could be observed. While endometrioid tumor (n = 19) showed a median IRS of 4, clear cell carcinoma (n = 10) showed a median IRS of 3.5, serous carcinoma a median IRS of 3 (n = 89), and mucinous carcinoma (n = 10) a median IRS of 2.5 (p = 0.009), as shown in Figure 2. On n = 28 slides, staining was not successful, or no ovarian cancer tissue was gained.
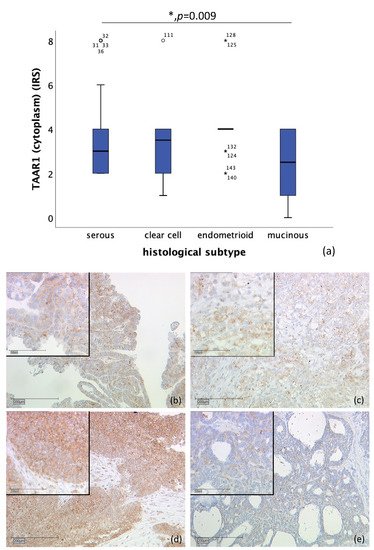

Figure 2. Correlation of cytoplasmic TAAR1 expression with histological subtype (p = 0.009) (a) boxplot of cytoplasmic TAAR1 expression and histological subtype (b) serous carcinoma (n = 89) with a cytoplasmic TAAR1 IRS of 3, magnification ×10 and ×25 in the inset (c) clear cell carcinoma (n = 10) with a cytoplasmic TAAR1 IRS of 3, magnification ×10 and ×25 in the inset (d) endometrioid carcinoma (n = 19) with a cytoplasmic TAAR1 IRS of 4, magnification ×10 and ×25 in the inset (e) mucinous carcinoma (n = 10) with a cytoplasmic TAAR1 IRS of 2, magnification ×10 and ×25 in the inset; * p < 0.05 was considered statistically significant.
3. TAAR1 Expression in High-Grade and Low-Grade Serous Ovarian Cancer
n = 110 tissue sections from patients diagnosed with serous ovarian carcinoma were stained (high-grade, n = 80; low-grade, n = 24; unknown, n = 6). Due to missed hits of ovarian cancer tissue and/or failed staining in n = 17 cases, n = 93 slides were examined. Correlation of membrane TAAR1 expression, with grading of serous carcinoma, showed that TAAR1 is expressed significantly higher in low-grade serous carcinoma (median IRS of 4; n = 22) compared to high-grade serous carcinoma (median IRS of 3; n = 71) (p = 0.028) (Figure 3).
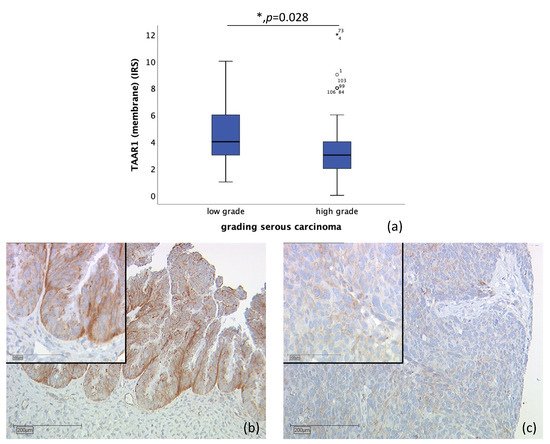

Figure 3. Correlation of membrane TAAR1 expression with grading in serous carcinoma (p = 0.028) (a) boxplot of membrane TAAR1 expression and grading in serous carcinoma (b) low grade serous carcinoma (n = 22) with a membrane TAAR1 IRS of 4, magnification ×10 and ×25 in the inset (c) high-grade serous carcinoma (n = 71) with a membrane TAAR1 IRS of 2, magnification ×10 and ×25 in the inset; * p < 0.05 was considered statistically significant.
