1. Introduction
Dietary recommendations suggest that fat should represent 20% to 35% of energy intake and the type of fat ingested is a fundamental clue in the prevention of several diseases
[1]. Indeed, fat overconsumption and fat quality have been linked to obesity, insulin resistance and the metabolic syndrome
[2]. Dietary fats and oils are composed of triglycerides made of three fatty acids esterified on a glycerol backbone. As fatty acids are highly diverse, the classifications of fatty acids are based on their carbon chain length, the number and the position of the unsaturations (double bounds) present between two carbons. They are classified into different families: saturated FA (SFA), with no unsaturation such as palmitic or stearic acids, monounsaturated (MUFA)—if it contains one double bond—and polyunsaturated (PUFA)—if there are more than one. The palmitoleic acid (16:1) and the oleic acid (18:1) represent two important MUFA. The PUFAs can further be subdivided into the two renowned omega-6 (n-6) (linoleic acid, arachidonic acid) and omega-3 (n-3) acids (α-linolenic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA)) based on the distance from the first double bond to the methyl end, although PUFA n-7 and n-9 exist
[3]. Both omega-6 and omega-3 are essential fatty acids and must be obtained exclusively from the dietary sources.
According to conventional dietary recommendations, a healthy diet should include a daily consumption of fats: an inadequate quantity of fat might increase the risk of conditions such as depression, psoriasis, Alzheimer’s disease and schizophrenia
[4][5][4,5]. To date, several edible oils have been characterized for their health benefits, particularly due to their abundance in MUFA and/or PUFA. Extra-virgin olive oil (EVOO), sunflower oil (SFO), palm oil, and fish oil have been extensively studied for their contribution to disease prevention. However, other types of edible oils may play a role in preserving health status. For instance, due to their high concentration in MUFA and PUFA and a low quantity of SFA, mustard and canola oils display beneficial effects on lipid serum profile and the cardiovascular system
[6][7][6,7].
EVOO represents the main source of fat in the Mediterranean diet
[8]. It is constituted by a high content of MUFA (mostly oleic acid), a variable but significant amount of PUFA (ranging from 1.5% to 21%), and minor amounts of antioxidant micronutrients such as polyphenols, squalene, lignans, phenyl-ethyl alcohols and secoiridoids
[9][10][9,10]. Oleic acid (18:1 n-9) represents 49% to 83% of total FA in olive oil
[11] and its consumption has been related to improved pancreas and liver secretory activity as well as reduced gastric-duodenal ulcers risk
[12]. Moreover, MUFAs are able to modify plasma lipids and lipoprotein composition and hence reduce inflammation, oxidative stress and coagulation and ameliorate glucose homeostasis and blood pressure
[12]. This evidence has confirmed the beneficial properties of EVOO bioactive components that have led to the established health claims by FDA and EFSA. Both agencies stated that oleic acid contained in olive oil, together with the present polyphenols, contributes to the maintenance of normal blood cholesterol levels
[13][14][13,14].
The SFO contains a large amount of PUFA (linoleic acid 60% to 70%), but MUFA (oleic acid), SFA (stearic acid) and tocopherol are present as well in adequate quantities
[15][16][15,16]. This oil has traditionally been used as control in several studies
[15][17][15,17]. It has been shown that SFO can modify the serum lipid profile by reducing total cholesterol and low-density lipoprotein (LDL) levels
[18][19][18,19]. On the other hand, a diet rich in linoleic acid increases reactive oxygen species (ROS) production compared to a SFA-rich diet, thereby promoting lipid peroxidation
[2].
Different from the previous two fatty acids, fish oil is a good source of the B12 and D vitamins and omega-3 FAs (EPA and DHA)
[20][21][20,21]. The favourable health effects of fish oil were initially established by the remarkably low incidence of coronary artery disease within the Inuit community, despite consuming a high-fat diet
[22]. These health effects have largely been attributed to EPA and DHA. Indeed, several studies have reported that diet supplementation with purified EPA and DHA for more than three years result in cardioprotective effects
[23][24][23,24]. In the past years, several meta-analyses and systematic reviews have been published in order to evaluate the association between fish oil and protection from cardiovascular risk
[25][26][27][28][29][30][31][32][25,26,27,28,29,30,31,32]. Most of the studies reported no significant association of omega-3 supplementation with fatal or nonfatal coronary heart disease or any major vascular events. Furthermore, a large cohort study recently demonstrated that diet supplemented with fish oil containing omega-3 failed to reduce the incidence of major cardiovascular events or cancer
[33]. If omega-3 FA supplement containing EPA and DHA is approved from FDA, it is not the same for the other dietary supplements of fish oil. Different from the first, fish oil supplement can include other fats and cholesterols as well, which may negatively affect the health response
[34].
Palm oil is constituted of 51% SFA (palmitic acid), 38% MUFA, 11% PUFA
[35], carotenoids, lycopene and xanthophylls
[36]. Rats fed with 12% palm oil for one-year display increased total serum cholesterol levels, but lower aortic accumulation of cholesteryl esters compared to SFO, soybean and butter most likely due to the high concentration of micronutrients and MUFA in this oil
[18]. Moreover, palm oil may reduce oxidative stress associated to ischemia-reperfusion injury
[37]. Further studies are needed to clarify the contribution of these edible oils on human health, evaluating the role played by different fatty acids.
It is becoming evidently clear that the various dietary oils differ not only in the FA profile, but in the content of non-saponifiable species as well. Studies on olive oil have regularly been more focused on these non-saponifiable components. On the contrary, the direct role of oleic acid has been neglected. Different from other FA, oleic acid is not an essential FA, since it can be de novo synthesized by stearoyl-CoA desaturase 1 (SCD1). Exploring SCD1, the master regulator of oleic acid synthesis, may therefore offer important insights on the role of the oleic acid largely present in olive oil. In this light, in vivo and in vitro studies in which the activity of SCD1 has been shut down represent a valid opportunity to evaluate the direct contribution of oleic acid supplementation with the diet (i.e. EVOO). To date, several clinical trials are currently conducted focusing on SCD1 (Table 1) aiming to understand how nutrients may interact with the organism, regulating energy and metabolic homeostasis as well as disease progression.
Table 1. Stearoyl-CoA desaturase 1 (SCD1) clinical trials.
|
| Trial Identifier |
|
| Trial Phase (Status) |
|
| Title |
|
| Intervention |
|
|
| NCT02647970 |
|
| Completed |
|
| Stearoyl-CoA Desaturase and Energy Metabolism in Humans |
|
| Behavioral: PUFA‒Cys/Met diet |
| Behavioral: SFA+Cys/Met diet |
|
|
| NCT03572205 |
|
| Completed |
|
| Fatty Acid Desaturase Gene Locus Interactions with Diet (FADSDIET2) |
|
| Dietary Supplement: LA |
| Dietary Supplement: ALA |
|
|
| NCT03282253 |
|
| Not yet recruiting |
|
| Elevated Stearoyl-CoA Desaturase-1 Expression Predicts the Disease Severity of Severe Acute Pancreatitis |
|
|
|
| NCT02543216 |
|
| Completed |
|
| Gene–Diet Interactions in Fatty Acid Desaturase 1 Gene |
|
| Dietary Supplement: Sunflower oil |
|
|
| NCT03842891 |
|
| Completed |
|
| Genetic Variants Modulate Association Between Dietary n-3 LCPUFAs and DHA Proportion in Breast Milk |
|
|
|
| NCT01661764 |
|
| Completed, Has Results |
|
| Fish Oil Supplementation, Nutrigenomics and Colorectal Cancer Prevention |
|
| Drug: Eicosapentanoic acid and docosahexanoic acid |
| Drug: Oleic Acid |
|
|
| NCT02337231 |
|
| Completed |
|
| Botanical Oils Study to Determine Genetic Differences in the Way Your Body Processes Fats in Edible Oils |
|
| Dietary Supplement: soybean oil and borage oil |
|
Lipids ingested with the diet are primarily digested and absorbed in the small intestine, followed by delivery to their sites of action in the body by crossing the liver. Thus, the gastrointestinal tract and the liver are at the nexus between a vast source of nutrients and the rest of the body and are connected both anatomically and functionally. The gut-liver axis is characterized by bidirectional traffic: nutrients and factors derived from gut lumen reach the liver through the portal circulation, while bile acids are released in the small intestine from the biliary duct.
In this review, we will particularly focus on the role of the major component of olive oil—the oleic acid. We will exploit the contribution of SCD1 and its product oleic acid in health and diseases, with particular attention to the gut-liver axis. Although it might appear controversial in some cases, it is evidently clear that oleic acid plays an essential part in the development and the homeostasis of our organism, and possible disruption of its pathway may result in disease onset and progression.
2. Oleic Acid in Health and Disease
In order to characterize the role of oleic acid in health and disease, numerous studies and clinical trials have been conducted (
Table 2). Several studies have highlighted the importance of MUFA in the diet, suggesting that the source and origin of MUFA is fundamental to analyse the beneficial effects of this FA
[38]. In Greece, Italy and Spain, olive oil consumption constitutes approximately 60% of MUFA intake, whereas in other countries MUFAs are mainly introduced with meat products
[39]. A correlation between MUFA intake and the reduction of cardiovascular heart disease risk have been described
[40], whereas a subsequent meta-analysis observed no significant association
[41]. These conflicting data could possibly be explained considering the different sources of MUFAs consumed by different cohorts of patients. Llorente-Cortes et al. demonstrated that a MUFA-rich diet reduced the postprandial monocytes inflammatory state linked to metabolic syndrome
[42]. Indeed, MUFAs display beneficial effects on insulin sensitivity and type 2 diabetes mellitus
[43]. In particular, Vessby et al. observed that the reduction of SFA and the concomitant addition of MUFA to the diet ameliorated insulin sensitivity
[44]. The western diet is rich in foods with high SFA abundance such as red meat and processed foods
[45]. This fat overload contributes to weight gain and the succeeding inflammation, and SFA induce insulin resistance and type 2 diabetes mellitus
[46]. Palmitic acid promotes the synthesis of non-esterified fatty acids (NEFAs), ceramides and reactive oxygen species (ROS), mitochondrial dysfunction and inflammation
[47][48][47,48]. SFA are able to alter the microbiota composition in the gut as well by up-regulating LPS and toll-like receptor 4 (TLR4) levels
[49]. The substitution of palmitic with oleic acid in the diet is able to reverse these detrimental metabolic effects of SFA. Cell culture studies have facilitated the definition of these molecular metabolic changes. In detail, oleic acid enhances mitochondrial oxidation of SFA by increasing triacylglycerol (TAG) and by reducing diacylglycerol (DAG) and ceramide production, thus protecting the cells from inflammation
[50].
Table 2. Oleic acid clinical trials.
|
| Trial Identifier |
|
| Trial Phase (Status) |
|
| Title |
|
| Intervention |
|
|
| NCT00715312 |
|
| Completed |
|
| Effect of Oleic Acid on Inflammation Markers and Blood Lipid Metabolites: A Randomised, Double-Blind, Crossover Study |
|
| Novel Olein |
|
|
| NCT01042340 |
|
| Completed |
|
| Energy Dense Oleic Acid Formula to Geriatric Patients |
|
| Calogen®–an energy dense oleic acid-based formula |
|
|
| NCT01124487 |
|
| Completed |
|
| The Acute Effects of Oleic Acid Enriched diets on Lipids, Insulin Sensitivity and Serum Inflammatory Markers |
|
| Dietary Supplement: The acute effects of dietary fat on lipid profile, insulin sensitivity and inflammatory markers |
|
|
| NCT02029833 |
|
| Completed |
|
| Canola Oil Multi-Centre Intervention Trial II |
|
| Other: Regular Canola Oil |
| Other: High Oleic Canola Oil |
| Other: Western Type Diet–Common Dietary Oils |
|
|
| NCT03054779 |
|
| Completed |
|
| Canola Oil Multi-Centre Intervention Trial II |
|
| Other: Canola Oil |
| Other: High oleic acid canola oil |
| Other: Western diet oil combination |
|
|
| NCT02993380 |
|
| Completed |
|
| Effect of Olive Oil on Erythrocyte Membrane Fatty Acid Contents in Hemodialysis Patients |
|
| Dietary Supplement: Stir-fried olive oil group |
| Dietary Supplement: Natural olive oil group |
|
|
| NCT00529828 |
|
| Completed |
|
| Health Effects of CLA Versus Industrial Trans Fatty Acids |
|
| Procedure: Consumption of CLA enriched food |
|
|
| NCT01259999 |
|
| Completed |
|
| Energy Dense Formula to People Living in Old Peoples Home |
|
| Dietary Supplement: Calogen extra strawberry |
|
|
| NCT00059254 |
|
| Completed |
|
| Differential Metabolism of Dietary Fatty Acids |
|
| Dietary Supplement: Oleic acid (OA) |
| Dietary Supplement: Palmitic Acid (PA) |
|
|
| NCT01996566 |
|
| Completed |
|
| Fatty Acid Taste thresholds: Caproic, Lauric, Oleic, Linoleic, Linolenic |
|
Furthermore, oleic acid displays the ability to prevent SFA-induced inflammation. In high fat diet fed mice, oleic acid administration ameliorated insulin sensitivity, reduced pro-inflammatory cytokines levels as Interleukin-1β, Interleukin-6 and Tumor Necrosis Factor-α and up-regulated the anti-inflammatory Inteleukin-10 and adiponectin levels
[51]. Through this mechanism, oleic acid promotes M2 anti-inflammatory macrophages phenotype, leading to reduction of leukotriene B4 secretion and PTEN expression
[52].
The peculiar beneficial role of oleic acid has been observed in several diseases including coronary heart disease, rheumatoid arthritis, and cancer. Oleic acid consumption prevents the risk of developing rheumatoid arthritis by increasing leukotriene A3 levels, a potent inhibitor of pro-inflammatory LTB4
[53]. In colorectal cancer HT-29 cells, oleic acid promotes apoptosis and cell differentiation via the downregulation of cyclooxygenase and Bcl-2 expression
[54]. In breast cancer cells, oleic acid reduces the expression of the oncogene Her-2/neu and acts synergistically with anticancer drug trastuzumab
[55]. Moreover, in breast tissue cells, oleic acid reduces the entering of lipid peroxidation into the phospholipid membrane of the cells
[56].
Interestingly, in animal models, oleic acid is able to induce lung injury miming acute respiratory distress syndrome (ARDS). Oleic acid administration induces direct toxicity to the endothelial cells characterized by endothelial necrosis, epithelial injury and neutrophil infiltration
[57][58][57,58]. Recently, it has been demonstrated that Liver X Receptor (LXR) activation protects the lungs from oleic acid-induced ARDS by decreasing the inflammatory response and by promoting antioxidant capacity
[59]. On the other hand, in lung cancer, oleic acid exerts beneficial effects through promotion of apoptosis, mitosis arrest and cellular differentiation and by inhibiting angiogenesis
[60]. In murine models, Piegari et al. have shown that an oleic acid-enriched diet ameliorated animal survival and lung tumour latency, confirming the oleic acid anticancer properties
[61].
Finally, oleic acid displays a pivotal role in the development of the brain, the organ with the highest lipid content of the body second to white adipose tissue. In this organ, lipids are essential for the correct homeostasis and alterations in lipid metabolism are linked to neurological diseases
[62]. Oleic acid is the only FA synthesized by astrocytes and it acts as a neurotrophic factor for neurons
[63]. In astrocytes, albumin uptake and transcytosis via endoplasmic reticulum induces sterol regulatory element-binding protein-1 (SREBP-1) and stearoyl-CoA desaturase expression, causing oleic acid production. The synthesized oleic acid promotes axonal and dendrite growth and induces doublecortin expression, resulting in neuron migration
[64][65][66][64,65,66]. These data confirm the pivotal role of oleic acid in astrocyte-neuron crosstalk and further studies should be performed in order to evaluate the role of oleic acid in neurodegenerative disorders. Given the crucial role of oleic acid in brain metabolism, Priore et al. treated C6 glioma cells with oleic acid and hydroxytyrosol and observed a reduction in de novo fatty acid and cholesterol synthesis, a crucial step in human glial cells malignancy
[67][68][67,68].
3. SCD1: The Oleic Acid Producer
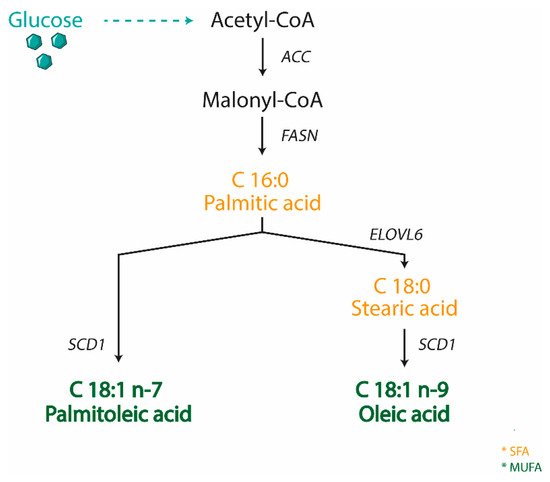
SCD is an enzyme anchored to the endoplasmic reticulum, where it catalyzes the biosynthesis of MUFA from SFA, either derived from the diet or synthesized de novo. SCD is Δ9-fatty acyl CoA desaturases that catalyzes the formation of a double bond in the cis-delta-9 position of saturated fatty acyl CoA. The preferred substrates of SCD are palmitic (C16:0) and stearic (C18:0) acids, which are converted into palmitoleic (C16:1 n-7) and oleic (C18:1 n-9) acids, respectively
[69] (
Figure 1). These products are the most abundant MUFA incorporated into different lipid species, including triglycerides, wax esters, cholesterol esters, and phospholipids
[70]. The ratio of SFA to MUFA is important to modulate phospholipid composition, and an unbalanced ratio toward SFA production is linked to multiple pathological conditions including obesity, diabetes, cardiovascular and neurological diseases, and cancer
[71]. Moreover, MUFAs serve as mediators of signal transduction and cellular differentiation
[71]. Thus, the expression of SCD is highly important in both physiological and pathological conditions and SCD is consequently tightly controlled at both transcriptional and post-translational level. Numerous dietary and hormonal signals and several transcription factors such as LXR, SREBP1C, carbohydrate response element binding protein (ChREBP), peroxisome proliferator activated receptor (PPAR), and estrogen receptor (ER) are involved in SCD transcriptional control
[72][73][72,73].
Figure 1. Scheme representing the biosynthesis of MUFA in animals. Stearoyl CoA Desaturase 1 (SCD1) catalyzes the rate-limiting step for the conversion of saturated fatty acid (SFA) into monounsaturated ones (MUFA). (ACC—acetyl CoA carboxylase; FASN—fatty acid synthase; ELOVL6—fatty acid elongases 6).
SCD is a highly conserved enzyme with multiple isoforms sharing similarities in protein sequences
[74]. To date, two human isoforms (1 and 5) and four mouse SCD isoforms (SCD1-4) have been identified
[75][76][75,76]. Despite a distinct distribution pattern, the different isoforms share the equal enzymatic function. Particularly, in both human and mice, SCD1 represents the predominant isoform and it is ubiquitously expressed among tissues, with constitutively high levels in lipogenic tissues, such as liver and white adipose tissue
[74]. Contrarily, SCD5 is unique to primates and is highly expressed in brain and pancreas
[76]. In adult mice, Scd2 isoform is ubiquitously expressed in most tissues except for the liver, whereas Scd3 and Scd4 expression is more restricted
[77][78][79][77,78,79].
The existence of multiple SCD1 isoforms, sharing a high sequence homology and catalyzing the same biochemical reaction, poses difficulties in order to distinguish the role of each single isoform and its metabolic contribution. To this end, substantial insights into SCD1 functions have been gained by the use of specific mouse models, in which the expression of SCD1 is downregulated or its activity is inhibited. Several genetically engineered whole body and tissue specific SCD1 knockout models have been utilized
[80] as well as Asebia mice, characterized by a whole-body deficiency of SCD1 due to a spontaneous mutation within the SCD1 gene
[81]. Additionally, mice treated with antisense oligonucleotides (ASO) against SCD1—which reduces SCD1 expression in liver and adipose tissue—have been extensively employed
[82]. Finally, various selective inhibitors targeting SCD1 activity have enabled the clarification of the role of this enzyme. Overall, these models demonstrate that SCD1 is a master regulator of lipid metabolism and is deeply involved in body weight regulation
[83][84][83,84]. This is specifically highlighted by the resistance to diet-induced obesity and hepatic steatosis observed in SCD1 deficient mice
[85]. These effects are predominantly derived from an increase in FA oxidation and thermogenesis, as well as a reduced lipid synthesis. The increased FA oxidation is mainly explained by a defect of skin permeability in SCD1KO mice which is causing heat leak and water link. The increased lipid oxidation is mediated by the induction of the AMP-activated protein kinase (AMPK)
[86], resulting in phosphorylation and inactivation of ACC. The inactivation of this enzyme reduces the cellular levels of malonyl-CoA, a substrate for FA biosynthesis, which in turn suppresses FA oxidation by inhibiting the mitochondrial carnitine palmitoyltransferase 1 (CPT1) shuttle system, which controls the import and oxidation of FA in mitochondria. Malonyl-CoA reduction in SCD1KO mice relieves the inhibition of CPT1 by directing FA into mitochondria where they are subsequently oxidized. In SCD1KO mice, the up-regulation of whole-body thermogenesis in brown adipose tissue (BAT) is mediated by the activation of PGC-1α and uncoupling protein-1, which uncouples oxidative respiration from ATP synthesis, resulting in dissipation of energy as heat
[87]. These modulations result in an increased rate of basal thermogenesis and, consequently, of whole-body energy expenditure.
Although many efforts have been made utilizing total body SCD1KO mice, the confirmation that these mice display altered skin permeability enhancing energy expenditure and protection from high fat diet-induced obesity
[88], has led to the generation of tissue specific SCD1KO mice in order to study the contribution of this enzyme to metabolic disorders and related diseases.
4. SCD1 in the Gut-Liver Axis
The liver plays a central role as a metabolic hub, where multiple biochemical processes converge to render food nutrients available to the rest of the body. However, an unbalanced diet—rich in lipid and carbohydrates—together with a sedentary lifestyle, may severely compromise the hepatic health status. Indeed, it has been shown that a high energy intake with a concomitant low energy expenditure resulted in hepatic lipid accumulation, one of the key features of non-alcoholic fatty liver disease (NAFLD). NAFLD is the most common disease of the Western Countries, characterized by a spectrum of conditions that vary from hepatic steatosis to severe form of non-alcoholic steatohepatitis (NASH), which may eventually progress toward cirrhosis and end-stage liver diseases
[89][117]. Despite the large amount of studies focused on the impact of hepatic metabolic alterations leading to the toxic effects of excess lipids and promotes liver diseases, it is now clear that metabolites from other organs may account for disease progression, such as adipose tissue and gut, as well. Whereas the communication between the adipose tissue and the liver has been extensively investigated, the gut-liver cross-talk exploration is just at the beginning. Not only the liver can influence the gut phenotype via bile acids or metabolites, but the gut harboring microbiota and secretory factors as well can affect the hepatic health status and promote lipid accumulation. However, accumulating data show that the total amount of lipids is not the major determinant of lipotoxicity, but that specific classes of lipids (palmitic acid, cholesterol, ceramides) promote cellular damage and disease progression
[90][118].