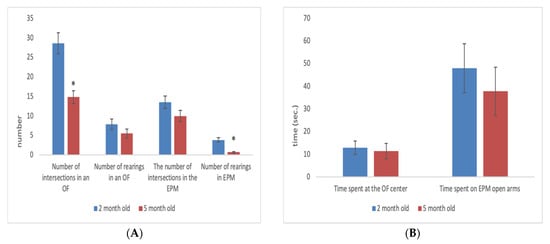
Rats are considered adults from 2 to 5 months. During this period, they are used for experimentation in physiology and pharmacology. Adult rats, depending on their age, can be in a different physiological state, which can influence the results of experiments carried out on them. Despite this, age-related changes in adult rats have not yet been examined. Our results showed that as male and female rats progressed from 2 to 5 months of age there was a decrease in the level of motor and exploratory activities, and an increase in the level of anxiety-like behaviour. Age-related changes were dependent upon initial individual characteristics of behaviour. For example, animals that demonstrated high motor activity at 2 months become significantly less active by 5 months, and animals that showed a low level of anxiety at 2 months become more anxious by 5 months. Low-activity and high-anxiety rats did not show any significant age-related changes from 2 to 5 months of age. The results of this work should be taken into account when choosing the age of rats for conducting behavioural experiments.
- age
- behaviour
- open field
- physical activity
1. Introduction
2. Age-Related Changes in Adult Rats
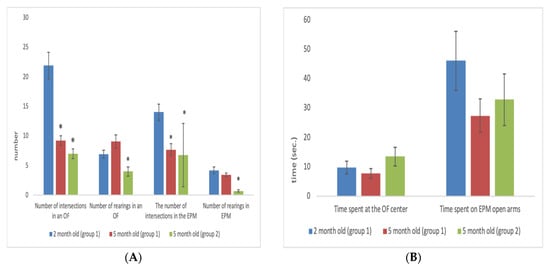
3. Age-Related Changes in Rats with High and Low Motor Activity in the OF and EPM Tests
In active females, motor activity decreased with age in the OF test (U = 0.01; Z = 3.06; p = 0.002). However, these changes were less pronounced than in males. In females that were passive in the OF, only a decrease in the number of rearings in the EPM was noted (U = 7.50 Z = 2,108,293 p = 0.035006)
The active and passive males in the OF test at the age of 2 months differed only in the level of horizontal (U = 0.01; Z = −3.07; p = 0.02) and vertical activity (U = 0.01; Z = −3.06; p = 0.002) (Table 1). At the age of 5 months, these differences diminished. In active and passive females at the age of 5 months, the differences observed at 2 months in the level of motor activity in the OF (U = 0.01; Z = −3.06; p = 0.002) also receded (Table 2).
In males that were active in the EPM, a significant decrease with age in motor activity was also observed, both in the OF (U = 0.01; Z = 3.06; p = 0.002) and in the EPM (U = 0.01; Z = 3.06; p = 0.002). The number of rearings also decreased in the OF (U = 8; Z = 2.04; p = 0.04) and in the EPM (U = 1.0; Z = 2.9; p = 0.003) tests. There were no age-related differences in the level of anxiety.
In males that were passive in the EPM, no significant age-related changes in the levels of motor and exploratory activities were
In females active in the EPM, motor activity significantly decreased with age in the OF (U = 7.50; Z = 2.11; p = 0.03) and EPM (U = 1; Z = 2.94; p = 0.003) tests, and the number of rearings in the EPM decreased (U = 2; Z = 2.81; p = 0.005). No significant age-related changes were observed in passive females
Males, both active and passive in the EPM at the age of 2 months significantly differed in the number of intersections of the maze compartments (U = 0.01; Z = −3.07; p = 0.002). At 5 months, these differences were not apparent.
EPM motor activity in females at 2 months was significantly greater compared to passive females (U = 0.01; Z = −2.93; p = 0.003). They also spent longer time in the open arms (U = 1.50; Z = −2.71; p = 0.007) than rats that were passive in the EPM. At 5 months, these differences between active and passive females in the EPM were not apparent.
4. Age-Related Changes in Rats with High (HA) and Low Levels of Anxiety (LA) in the OF and EPM Tests
Despite the fact that we did not reveal age-related differences in the level of anxiety in the general population of Wistar rats, LA in the OF test males, at the age of 2 months, demonstrated a low level of anxiety (spent a longer time in the centre of the OF), and had a significantly increased level of anxiety by the fifth month (U = 11.50; Z = 2.10; p = 0.036). In addition, in LA males in the OF, by the fifth month, motor activity decreased both in the OF (U = 0.01; Z = 3.31; p = 0.0009) and in the EPM (U = 7; Z = 2.57; p = 0.01) tests, and the number of rearings in the OF (U = 8.50; Z = 2.41; p = 0.02) and in the EPM (U = 8; Z = 2.47; p = 0.01) also decreased (Table 5). Those males that demonstrated a high level of anxiety at age 2 months had no reliable age-related changes in levels of motor activity and anxiety. We observed only a significant decrease in the number of rearings in the EPM (U = 3.0; Z = 2.68; p = 0.007).
In both LA and HA in the OF test females, no significant age-related changes in the studied parameters were observed, except for a decrease in the number of rearings in the EPM in calm (U = 3.5; Z =2.62; p =0.009) and in anxious animals (U =7.5; Z = 2.11; p = 0.035)
The observed significant differences between LA and HA in the OF test in males at the age of 2 months in motor activity (U =1; Z = −3.07; p = 0.002), the number of rearings (U =5.5; Z = −2.55; p = 0.01), and the time spent in the centre of the arena (U = 0.01; Z = −3.18; p = 0.001) was not present by the fifth month. The observed significant differences between LA and HA 2-month-old females in terms of the time spent in the centre of the OF (U = 0.01; Z = −3.07; p = 0.002) was also not apparent at the age of 5 months.
Males who, at the age of 2 months, demonstrated a low level of anxiety in the EPM (prolonged time spent in open arms), by 5 months significantly reduced the time spent on open arms (U = 7.0; Z = 2.17; p = 0.03). Motor and exploratory activity of LA in EPM males, both in the EPM (U = 7.5; Z = 2.11; p =0.035) and in the OF (U = 1; Z = 2.94; p = 0.003) also significantly decreased with age. In males that demonstrated a high level of anxiety at the age of 2 months, the number of rearings in the EPM decreased with age (U = 2.5; Z = 2.75; p = 0.006). No other significant age-related changes in behaviour were observed In males that demonstrated a high level of anxiety in the EPM at the age of 2 months, practically no significant age-related changes in behaviour were observed.
Females showing a low level of anxiety in the EPM at the age of 2 months also significantly reduced the time spent on open arms by 5 months (U = 4.0; Z = 2.555; p = 0.01). In addition, with age, they had a decrease in the number of rearings in the EPM (U = 2.5; Z = 2.75; p = 0.006). In females showing a high level of anxiety in the EPM, by the fifth month the time spent in the open arms did not change. However, the number of rearings in the EPM significantly decreased (U = 3.0; Z = 2.68; p = 0.007)
Males with high and low anxiety in the EPM differed at the age of 2 months in the duration of stay in the open arms of the maze (U = 0.01; Z = −3.07; p = 0.002). By the fifth month, significant differences between groups were not observed.
Females with high and low anxiety in the EPM at 2 months significantly differed in the time spent on the open arms of the maze (U = 0.0 1; Z = −3.07; p = 0.002), as well as in the level of motor activity in the EPM (U = 3; Z = −2.68; p = 0.007). These differences were not found at the fifth month.
5. Conclusions
- In male and female Wistar rats there is a decrease in the level of motor and exploratory activities from 2 to 5 months of age.
- Age-related changes in adult Wistar rats depend on their initial individual characteristics of behaviour.
- Animals that demonstrate high motor activity at 2 months become significantly less active by 5 months.
- Animals that show a low level of anxiety at the age of 2 months become more anxious by 5 months.
- Low-activity and high-anxiety rats do not exhibit age-related changes in OF and EPM tests from 2 to 5 months of age, except for a decrease in the number of rearings in the EPM.
References
- Trull, F.L.; Rich, B.A. More regulation of rodents. Science 1999, 284, 1463.
- Sengupta, P. The Laboratory Rat: Relating Its Age With Human’s. J. Prev. Med. 2013, 4, 624–630.
- Smith, J.R.; Bolton, E.R.; Dwinell, M.R. The Rat: A Model Used in Biomedical Research. Methods Mol. Biol. 2019, 2018, 1–41, doi:10.1007/978-1-4939-9581-3_1.
- Mashimo, T.; Serikawa, T. Rat resources in biomedical research. Pharm. Biotechnol. 2009, 10, 214–220, doi:10.2174/138920109787315105.
- Andreollo, N.A.; Santos, E.F.; Araújo, M.R.; Lopes, L.R. Rat’s age versus human’s age: What is the relationship? Bras Cir. Dig. 2012, 25, 49–51.
- Quinn, R. Comparing rat’s to human’s age: How old is my rat in people years? Nutrition 2005, 21, 775–777.
- Klein, Z.A.; Romeo, R.D. Changes in hypothalamic-pituitary-adrenal stress responsiveness before and after puberty in rats. Behav. 2013, 64, 357–363, doi:10.1016/j.yhbeh.2013.01.012.
- Ferguson, S.A.; Gray, E.P. Aging effects on elevated plus maze behavior in spontaneously hypertensive, Wistar-Kyoto and Sprague-Dawley male and female rats. Behav. 2005, 85, 621–628.
- Schulz, D.; Kouri, C.; Huston, J.P. Behavior on the water maze platform: Relationship to learning and open field exploration in aged and adult rats. Brain Res. Bull. 2007, 74, 206–215.
- Sotoudeh, N.; Namavar, M.R.; Zarifkar, A.; Heidarzadegan, A.R. Age-dependent changes in the medial prefrontal cortex and medial amygdala structure, and elevated plus-maze performance in the healthy male Wistar rats. IBRO Rep. 2020, 9, 183–194.
- Lomidze, N.; Zhvania, M.G.; Tizabi, Y.; Japaridze, N.; Pochkhidze, N.; Rzayev, F.; Gasimov, E. Age-related behavioral and ultrastructural changes in the rat amygdala. Neurobiol. 2020, 80, 433–442.
- Buechel, H.M.; Popovic, J.; Staggs, K.; Anderson, K.L.; Thibault, O.; Blalock, E.M. Aged rats are hypo-responsive to acute restraint: Implications for psychosocial stress in aging. Aging Neurosci. 2014, 6, 13.
- Lynn, D.A.; Brown, G.R. The ontogeny of anxiety-like behavior in rats from adolescence to adulthood. Psychobiol. 2010, 52, 731–739.
- Matzel, L.D.; Sauce, B. Individual differences: Case studies of rodent and primate intelligence. Exp. Psychol. Anim. Learn Cogn. 2017, 43, 325–340, doi:10.1037/xan0000152.
- King, G.; Scott, E.; Graham, B.M.; Richardson, R. Individual differences in fear extinction and anxiety-like behavior. Mem. 2017, 24, 182–190, doi:10.1101/lm.045021.117.
- Estanislau, C.; Ramos, A.C.; Ferraresi, P.D.; Costa, N.F.; de Carvalho, H.M.; Batistela, S. Individual differences in the elevated plus-maze and the forced swim test. Processes. 2011, 86, 46–51.
- Momeni, S.; Sharif, M.; Agren, G.; Roman, E. Individual differences in risk-related behaviors and voluntary alcohol intake in outbred Wistar rats. Pharmacol. 2014, 25, 206–215.
- Olton, D.S.; Markowska, A.; Breckler, S.J.; Wenk, G.L.; Pang, K.C.; Koliatsos, V. Individual differences in aging: Behavioral and neural analyses. Environ. Sci. 1991, 4, 166–172.
- Stam, R.; Croiset, G.; Akkermans, L.M.; Wiegant, V.M. Behavioural and intestinal responses to novelty in rats selected for diverging reactivity in the open field test. Brain Res. 1997, 88, 231–238.
- Markowska, A.L.; Stone, W.S.; Ingram, D.K.; Reynolds, J.; Gold, P.E.; Conti, L.H.; Pontecorvo, M.J.; Wenk, G.L.; Olton, D.S. Individual differences in aging: Behavioral and neurobiological correlates. Aging. 1989, 10, 31–43.
- Sandi, C.; Touyarot, K. Mid-life stress and cognitive deficits during early aging in rats: Individual differences and hippocampal correlates. Aging 2006, 27, 128–140.
- Balietti, M.; Pugliese, A.; Fabbietti, P.; Di Rosa, M.; Conti, F. Aged rats with different performances at environmental enrichment onset display different modulation of habituation and aversive memory. Learn Mem. 2019, 161, 83–91.
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Neurosci. Methods 1985, 14, 149–167.
- Arrant, A.E.; Schramm-Sapyta, N.L.; Kuhn, C.M. Use of the light/dark test for anxiety in adult and adolescent male rats. Brain Res. 2013, 256, 119–127.
- Boguszewski, P.; Zagrodzka, J. Emotional changes related to age in rats--a behavioral analysis. Brain Res. 2002, 133, 323–332.
- Parker, V.M.A. The socially isolated rat as a model of anxiety. Neuropharmacology 1986, 25, 663–664.
- Zelikowsky, M.; Hui, M.; Karigo, T.; Choe, A.; Yang, B.; Blanco, M.R.; Beadle, K.; Gradinaru, V.; Deverman, B.E.; Anderson, D.J. The Neuropeptide Tac2 Controls a Distributed Brain State Induced by Chronic Social Isolation Stress. Cell 2018, 173, 1265–1279.
- Donovan, M.; Mackey, C.S.; Platt, G.N.; Rounds, J.; Brown, A.N.; Trickey, D.J.; Liu, Y.; Jones, K.M.; Wang, Z. Social isolation alters behavior, the gut-immune-brain axis, and neurochemical circuits in male and female prairie voles. Stress. 2020, 13, 100278.
- Domènech-Abella, J.; Mundó, J.; Haro, J.M.; Rubio-Valera, M. Anxiety, depression, loneliness and social network in the elderly: Longitudinal associations from the Irish Longitudinal Study on Ageing (TILDA). Affect. Disord. 2019, 246, 82–88.
- Archer, J. Contrasting effects of group housing and isolation on subsequent open field exploration in laboratory rats. Sci. 1969, 14, 234–235.
- Heidbreder, C.A.; Weiss, I.C.; Domeney, A.M.; Pryce, C.; Homberg, J.; Hedou, G.; Feldon, J.; Cmoran, M.; Nelso, P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience 2000, 100, 749–768.
- Liu, Y.P.; Kao, Y.C.; Tung, C.S. Critical period exists in the effects of isolation rearing on sensorimotor gating function but not locomotor activity in rat. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1068–1073.
- Dalrymple-Alford, J.C.; Benton, D. Activity differences of individually and group-housed male and female rats. Learn. Behav. 1981, 9, 50–55.
- Grippo, A.J.; Ihm, E.; Wardwell, J.; McNeal, N.; Scotti, M.A.; Moenk, D.A.; Chandler, D.L.; LaRocca, M.A.; Preihs, K. The effects of environmental enrichment on depressive and anxiety-relevant behaviors in socially isolated prairie voles. Med. 2014, 76, 277–284.
- Bowman, R.E.; Beck, K.D.; Luine, V.N. Chronic stress effects on memory: Sex differences in performance and monoaminergic activity. Behav. 2003, 43, 48–59, doi:10.1016/s0018-506x(02)00022-3.
- Rojas-Carvajal, M.; Brenes, J.C.; Sequeira-Cordero, A. Age-dependent differences on neurochemistry and behavior in rats raised with low and high levels of maternal care. Brain Res. 2019, 372, 112054, doi:10.1016/j.bbr.2019.112054.
- Bowman, R.E.; Micik, R.; Gautreaux, C.; Fernandez, L.; Luine, V.N. Sex-dependent changes in anxiety, memory, and monoamines following one week of stress. Behav. 2009, 97, 21–29, doi:10.1016/j.physbeh.2009.01.012.
- Beery, A.K.; Holmes, M.M.; Lee, W.; Curley, J.P. Stress in groups: Lessons from non-traditional rodent species and housing models. Biobehav. Rev. 2020, 113, 354–372.
- Scholl, J.L.; Afzal, A.; Fox, L.C.; Watt, M.J.; Forster, G.L. Sex differences in anxiety-like behaviors in rats. Behav. 2019, 211, 112670.
- Ramos-Ortolaza, D.L.; Doreste-Mendez, R.J.; Alvarado-Torres, J.K.; Torres-Reveron, A. Ovarian hormones modify anxiety behavior and glucocorticoid receptors after chronic social isolation stress. Brain Res. 2017, 328, 115–122.
- Gouveia, A., Jr.; dos Santos, U.D.; Felisbino, F.E.; de Afonseca, T.L.; Antunes, G.; Morato, S. Influence of the estrous cycle on the behavior of rats in the elevated T-maze. Processes. 2004, 67, 167–171.
