The development of the mRNA vaccine against SARS-CoV-2 is unprecedented in the history of Vaccinology. The prototypes of the mRNA vaccine are BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna), which gained widespread use to prevent severe SARS-CoV-2 infection. As per CDC guidelines, these vaccines have undergone extensive testing and trials, and myocarditis and pericarditis occurred after the mRNA vaccines were not reported in trials. This paper reviews the published reports of mRNA vaccine-associated myopericarditis as of July 30, 2021, and provides insight into the possible Pathophysiologic mechanism; further research is required to understand long-term cardiac risks.
1. Introduction
The mRNA vaccines ((BNT162b2, Pfizer-BionTech, Pfizer, Inc.; Philadelphia, PA, USA) and Moderna (mRNA-1273, ModernaTX, Inc.; Cambridge, MA, USA)) demonstrated excellent safety and clinical efficacy profiles in clinical trials in adults in less than one year from the identification of the virus which is unprecedented in the history of vaccinology [1][2][1,2]. The mRNA vaccine used in the European Union is Comirnaty, which has the same mRNA, BNT162b2- as the Pfizer-BionTech, and is manufactured according to the same processes and procedures. The only difference is in their label. The mRNA COVID-19 vaccine Moderna is also known as Spikevax, which is used in the European Union. The Food and Drug Administration (FDA) issued emergency use authorization (EUA) on 11 December 2020, for Pfizer-BioNTech COVID-19 vaccine in persons aged > 16 years [3]. The European Medicine Agency (EMA) approved Comirnaty in people from 16 years of age on 21 December 2020. On 10 May 2021, the FDA expanded EUA to use the Pfizer mRNA COVID-19 vaccine for children 12–15 years [4]. The Center for Disease Control and Prevention (CDC) subsequently recommended on 12 May 2021, that persons 12 years and older should get a COVID-19 vaccine.
Post-vaccination myocarditis has been reported as early as 1957 after smallpox vaccination [5]. Analysis of the Vaccine Adverse Event Reporting System (VAERS) data between 2011 and 2015, where a total of 357,188 reports were reviewed and found 199 cases of myocarditis and pericarditis. Only the smallpox vaccine emerged with an expectedly strong correlation with myocarditis and pericarditis [6]. Previous findings should be interpreted with caution regarding limitations affecting the voluntary reporting system and may be underreported. Conversely, in the current era of heightened surveillance by the CDC’s vaccine safety data (VSD) working group and the Vaccine Related Biological Products Advisory Committee (VRBPAC) on immunization practices after COVID-19, and post-marketing surveillance by the vaccine producing companies in the setting of conditional marketing authorization, the reporting of probable myocarditis and pericarditis cases is significantly higher. Since April 2021, increased cases of myocarditis and pericarditis have been reported in the United States after mRNA COVID-19 vaccination, particularly in adolescents and young adults [7][8][9][10][11][12][7,8,9,10,11,12].
The American Academy of Pediatrics (AAP) and the American Heart Association (AHA) have endorsed CDC recommendations and reiterated the potential benefits of COVID-19 vaccination, which outweighs rare myocarditis or pericarditis risks and recommend the vaccination for anyone 12 years of age and older [13][14][13,14]. Very little published data on the incidence of mRNA vaccine-associated myocarditis and pericarditis exist except those reported in the media. We performed a systematic search of electronic databases (PubMed, Scopus, medRxiv and bioRxV) with a goal to publish the results in the context of expanding the vaccine target population using the terms “mRNA vaccine complications”, “heart inflammation with COVID-19 vaccine”, “impact of COVID-19 vaccine in children and young adults”, “myocarditis after COVID-19 vaccination” and “pericarditis after COVID-19 vaccination”, and “myopericarditis after COVID-19 vaccination”.
2. Diagnosis and Management
The common clinical presentation of COVID-19 vaccine-associated myopericarditis includes chest pain, fever, palpitation, shortness of breath, fatigue, nausea, vomiting, abdominal pain or unusual symptoms such as forceful or thumping heart beats. Common signs of myopericarditis include tachypnea, tachycardia, murmur, gallop, diminished pulses, hypotension, hepatomegaly, edema and signs of low cardiac output
[15][27]. The laboratory tests commonly used include testing to detect any viral causes of myocarditis, serum concentrations of an inflammatory marker (C-reactive protein, erythrocyte sedimentation rate) and cardiac biomarker (troponin, brain type natriuretic peptide), electrocardiogram, echocardiogram, CMR and serologic testing for SARS-CoV-2 antibodies. Historically, the diagnosis of myocarditis is confirmed by histologic criteria, including acute myocyte injury with inflammatory cells’ infiltration, especially lymphocytes
[16][28]. A paradigm shift in the diagnosis of myocarditis has occurred
[17][29]. According to an AHA statement, four strata of diagnosis of myocarditis in children are recommended: biopsy proven, clinically suspected, confirmed by CMR and possible myocarditis. The shift in diagnosis acknowledges advancements in CMR and improvement in identifying a constellation of clinical signs and symptoms supportive of myocarditis.
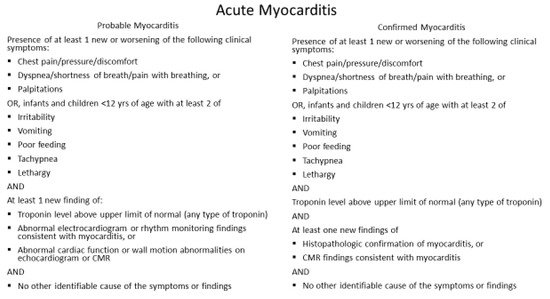
In most cases, COVID-19 vaccine-associated myocarditis is transient and self-limited; it is not justifiable to obtain an endomyocardial biopsy. Furthermore, the clinical impact of myocarditis varies widely due to the range of etiologies and the unpredictable physiologic responses depending upon the host’s response to the inciting agent. Elevated cardiac troponin may indicate acute cardiac injury, but not specific to the diagnosis of myocarditis, as many myocarditis patients are asymptomatic and are subclinical. Furthermore, features of myocarditis and pericarditis may overlap and commonly present as myopericarditis. Because of variable clinical manifestations of myocarditis, it is essential to follow the CDC definition of acute myocarditis (
Figure 1) and acute pericarditis (
Figure 2). Cardiac magnetic resonance imaging with tissue characterization using T1 and T2 mapping is a useful non-invasive modality for diagnosing myocarditis
[18][30]. Localized or generalized myocardial edema on T2 recovery images without evidence of late gadolinium enhancement (LGE) and no other clinical features can be the only CMR evidence of myocardial inflammation in mRNA COVID vaccine associated myocarditis in early stages. The AHA recommended “everyone to keep in touch with their primary care professional and seek care immediately if they have any of these symptoms in the weeks after receiving the COVID-19 vaccine: chest pain including sudden, sharp, stabbing pains; difficulty breathing/shortness of breath; abnormal heartbeat; severe headache; blurry vision; fainting or loss of consciousness; weakness or sensory changes; confusion or trouble speaking; seizures; unexplained abdominal pain; or new leg pain or swelling”
[14].
Figure 1. CDC working case definition for acute myocarditis. Modified from source:
http://www.cdc.gov; accessed 18 June 2021.
Figure 2. CDC working case definition for acute pericarditis. Modified from source:
http://www.cdc.gov; accessed 18 June 2021. * Typically described as pain made worse by lying down, deep inspiration, or cough and relieved by sitting up or leaning forward, although other types of chest pain may occur.
Acute myopericarditis associated with COVID-19 vaccine can be associated with arrhythmia. Anticipatory care includes judicious triaging of the disposition of patients seen in ambulatory or emergency setting for work-up or management of probable myopericarditis patients. Whenever possible, serology and PCR for common viral causes of myocarditis such as parvovirus B19, herpesvirus type-6, adenovirus, enterovirus, Epstein–Barr and cytomegalovirus should be obtained to rule out common causes of viral myocarditis. The treatment considerations for COVID-19 vaccine-associated myopericarditis include anti-inflammatory medications and guideline-directed medical therapy if left ventricular function is reduced
[19][31]. No data for any specific treatment for vaccine-associated myocarditis are available. Steroids are used for their potent anti-inflammatory action in cases where the patient has continued symptoms and troponin leak even after NSAIDs. However, steroids and IVIG are also immunomodulatory and immunosuppressive agents and could reduce the specific immune response against SARS-CoV-2 triggered by the vaccine. Thus, the duration of steroids administration should be limited to the resolution of the symptoms or ventricular arrhythmias or the recovery of the LV function. Among 29 cases with a known outcome, all were discharged to their homes within 1 week, and all made full clinical recoveries. However, the long-term impact of myocardial inflammation following COVID-19 mRNA vaccine as detected on CMR, remains unknown and needs to be systematically evaluated. Further studies are required to elucidate the pathophysiology that underlies this complication to seek mitigation strategies and to delineate optimal therapy.
It is prudent to maintain regular follow-up of patients with COVID-19 vaccine-associated myocarditis patients especially those with documented inflammation on CMR. Pending publication of long-term outcome data after SARS-CoV-2 vaccine-related myocarditis, we suggest adherence to the current consensus recommendation to abstain from competitive sports for 3 to 6 months with re-evaluation before sports participation
[20][21][32,33].