Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Yang Liu.
The utilization of paclitaxel, camptothecin, rhein, curcumin, and other natural small molecular drugs with unique rigid backbone structures and modifiable multiple sites as building blocks to form gels by self-assembly has attracted widespread attention. The obtained low-molecular-weight supramolecular gel not only retains the general characteristics of the gel but also overcomes the shortcomings of natural drugs, such as poor water solubility and low bioavailability.
- natural drug
- low molecular weight supramolecular gel
- self-assembly
- hydrogel
- organogel
1. Introduction
The gel is a kind of soft material with a three-dimensional network structure formed by crosslinking of colloidal particles, small molecular substances, or polymer chains under certain conditions, which has attracted much attention in the biomedical area, such as drug delivery, tissue engineering, and biosensors [1,2,3][1][2][3]. According to the difference of internal solvents, the gel can be divided into hydrogel, organogel, ionogel, and so on [4,5][4][5]. Besides, according to the composition and formation mechanism, we can also divide the gel into macromolecular gel and supramolecular gel [6]. Among them, supramolecular gels are mostly formed from low-molecular-weight molecules, oligomers, or polymers through non-covalent interactions, such as electrostatic interaction, hydrogen bonding, van der Waals force, hydrophobic interaction, host–guest interaction, and so on, or dynamic covalent bonds [7,8,9][7][8][9]. Compared with macromolecular gels, supramolecular gels generally have the advantages of definite structural composition, easy chemical modification, lower critical gelation concentration, and reversible gel formation upon certain external stimulus, which have shown excellent prospects in the biomedical field [10,11,12][10][11][12].
With the further development of research, supramolecular gels formed from natural small molecular drugs have attracted much attention from researchers [13,14][13][14]. The natural small molecular drug is a kind of substance derived from biological metabolism, which generally refers to the effective components of traditional Chinese medicine, such as paclitaxel, camptothecin, rhein, curcumin, and so on. Because of its extensive pharmacophore, unique stereochemical structure, multiple sites for modification, and rich sources, it is considered to be one of the great repositories of new drug development [15,16,17][15][16][17]. However, most natural drugs also have some disadvantages, including poor water solubility and low bioavailability. To improve their therapeutic effect, researchers have developed various carriers for the delivery of natural drugs, such as nanoparticles, micelles, liposomes, and polymer gels [18,19,20][18][19][20]. Among them, polymer gels have attracted increasing research interest in natural drug delivery because of their outstanding biocompatibility, viscoelasticity, controlled and sustained drug release performance, and certain targeting properties [21,22,23][21][22][23]. Nonetheless, it also has some limitations, such as low drug loading, incomplete degradation, and so on. To solve these problems, some studies directly used natural drug molecules as building blocks to self-assemble into low-molecular-weight supramolecular gels, which are further used to deliver drugs to lesion sites and exert their efficacy. In 1977, Acree et al. [24] inadvertently found that cholesterol can be self-assembled in isopropanol to form a transparent gel in the process of determining the solubility of cholesterol in organic solvents, which may be the first found low-molecular-weight supramolecular gel based on natural drug. In 2009, Gao et al. [25] took the paclitaxel (PTX) as an example and firstly established a general preparation method of nanofiber supramolecular hydrogel drug delivery system based on hydrophobic drug molecules. In 2011, Bag et al. [26] discovered that betulinic acid could self-assemble to form gels in some organic solutions, which revealed the face of the first supramolecular gel based on a triterpene drug (Figure 1).
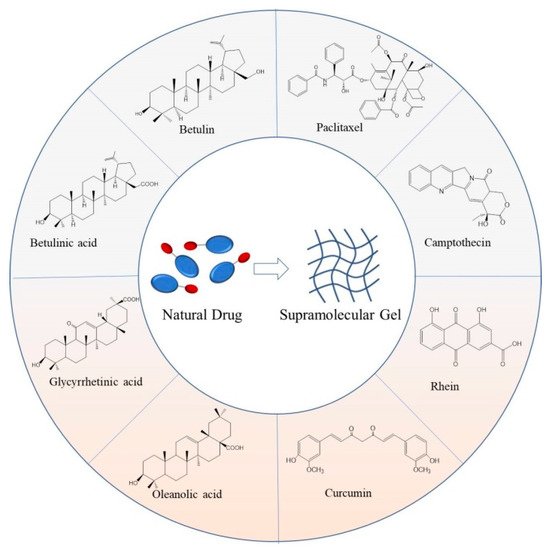
Figure 1. The structure schematic of reported representative natural drugs and the corresponding low-molecular-weight supramolecular gels formed from them.
2. Paclitaxel-Based Low-Molecular-Weight Supramolecular Gels
Paclitaxel (PTX) or taxol is a kind of tricyclic diterpenoid compound with high antitumor activity isolated from Taxus brevifolia. It is widely used in the treatment of breast cancer, ovarian cancer, and lung cancer, which is one of the most attractive anti-tumor star drugs in the clinical treatment [27]. Paclitaxel has a unique anti-tumor mechanism. By binding to tubulin and preventing its depolymerization, paclitaxel can effectively inhibit the mitosis of tumor cells, prevent the progress of the cell cycle, and then promote the apoptosis of tumor cells [28]. However, it is suffered from low water solubility, which seriously restricts the anti-tumor effect in vivo. Although a variety of paclitaxel-based preparations were put on the market or in the stage of clinical research, the construction of novel paclitaxel-based drug delivery systems is still one of the hotspots at home and abroad.
Short peptides have a large number of amino and carboxyl groups, which present good biocompatibility and rich structural diversity, and are often used to modify and regulate the hydrophilicity and hydrophobicity of natural small molecular drugs. Besides, through reasonable design, short peptides are easily assembled into hydrogels using non-covalent forces such as hydrogen bonding and π–π stacking, which have aroused extension attention in the construction of supramolecular gels. As mentioned above, the first paclitaxel-based low-molecular-weight nanofiber supramolecular hydrogel delivery system was obtained by the use of short peptides [25]. As shown in Figure 2, the author first introduced a carboxyl group into paclitaxel by esterification of C2′ hydroxyl group with succinic anhydride, and then a paclitaxel derivative with better solubility and water stability was obtained by amide condensation with a phosphatase substrate NapFFKYp short peptide. Under the stimulation of alkaline phosphorylase, the paclitaxel derivative can be dephosphorylated and further rapidly self-assembled to form a nanofiber hydrogel under non-covalent interaction. The obtained hydrogel could slowly release paclitaxel derivative with the same physiological activity as PTX and was able to inhibit the proliferation of HeLa cells. This study provided a simple and versatile formation method of hydrogel for enzyme-mediated self-assembly of hydrophobic drug molecules. Another study also found that the low concentration of paclitaxel released from this type of paclitaxel hydrogel not only enhanced neurite elongation as free paclitaxel did, but also promoted the branches of axons by inhibiting the depolymerization of tubulin which was not achieved by using free paclitaxel [29]. Furthermore, by simultaneously coupling doxorubicin [30], folic acid [31], dexamethasone [32], or embedding vorinostat [33] and tetrandrine [34] on short peptides, the researchers can further regulate the properties of paclitaxel-based supramolecular gels and improve the therapeutic effect. At the same time, through the accurate molecular design of the charge of the short peptide and the type of amino acid, the stability or dissociation of the formed paclitaxel-based hydrogel can be adjusted, and then the release of paclitaxel can be controlled, which can improve the anti-tumor effect and reduce the systemic toxicity [35].
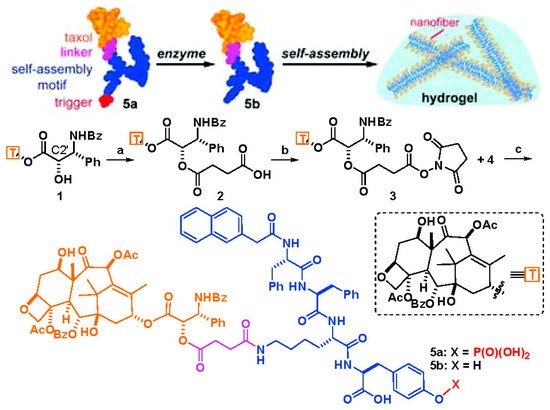
Figure 2. The schematic diagram of the formation of the first paclitaxel-derivative-based supramolecular nanofiber hydrogel obtained by the use of short peptides, and the preparation process of this paclitaxel derivative. Reprinted with permission from Copyright (2009) American Chemical Society.
3. Camptothecin-Based Low-Molecular-Weight Supramolecular Gels
Camptothecin (CPT) is an alkaloid active substance extracted from the traditional Chinese medicine Camptotheca acuminata with a quinoline ring as the basic core. Because of its inhibitory activity of DNA topoisomerase I, CPT is a commonly used broad-spectrum anticancer drug in the clinic [42][36]. Similar to paclitaxel, CPT has low water solubility and stability, making it difficult to use directly and safely in the body. To solve this problem, researchers often modify the active site on the structure of camptothecin (such as the hydroxyl group on the E-ring) to a certain extent and construct various new drug delivery systems to improve its solubility and anti-tumor activity [43][37].
The Cui group coupled two camptothecin molecules to the same short peptide and constructed a camptothecin analogue tubustecan (TT) [44][38]. Due to hydrogen bonding and other effects, tubustecan can cyclize and self-assemble to form tubular supramolecular nanostructures in an aqueous solution, which improves the water solubility of camptothecin and can be further used to load other hydrophobic drugs or molecules. In a representative study, they used a circular cell penetrating peptide iRGD to prepare a camptothecin-based tubustecan (TT6) through the coupling of biodegradable ethyl disulfonyl carbonate (etcSS linker) [45][39]. As shown in Figure 3, TT6 could first self-assemble to form a hollow tubular structure in an aqueous solution, and further assemble into a nanofiber hydrogel after the adding of PBS or DMEM medium. It was found that, when doxorubicin was embedded into the hydrogel, the resulting hydrogel could slowly release doxorubicin and TT6 with zero-order release characteristics. Besides, under the action of reductive GSH, TT6 could further quickly release CPT. In vivo experiments also showed that the gel can be formed quickly at the injection site, maintaining a sustained release of the drug for at least 45 days, and can improve the penetration and anti-tumor ability of the drug. When curcumin was introduced into this hydrogel, it was also found that it could inhibit the growth of primary breast cancer and prevent it from metastasizing to the lungs. The group also used TT as an immune enhancer for tumor immunotherapy based on the immune checkpoint blocker (ICBs) [46][40]. They used an amphipathic prodrug diCPT-PLGLAG-iRGD to prepare supramolecular gel. Because of the presence of PLGLAG polypeptide fragment, this hydrogel can be broken under the action of the over-expressed MMP-2 metalloproteinase in the tumor microenvironment, thus accelerating the release of CPT and the blended aPD1 antibody to induce the body to produce long-lasting anti-tumor immunity and thereby inhibit tumor recurrence and metastasis.
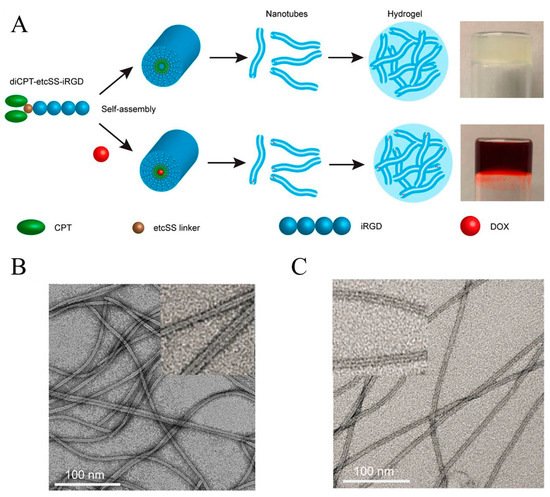
Figure 3. Schematic illustration of the camptothecin-based amphiphile tubustecan (TT6) hydrogel as chemotherapeutic carrier: (A) the chemical design, self-assembly, and drug loading of the hydrogel; (B) Representative TEM images of the hydrogel and (C) DOX-loaded hydrogel. Reproduced with permission from Copyright (2020) American Chemical Society.
4. Rhein-Based Low-Molecular-Weight Supramolecular Gels
Rhein (Rh), a kind of anthraquinone compound extracted from the traditional Chinese medicine rhubarb, has a wide range of pharmacological effects. It can inhibit the proliferation and induce apoptosis of malignant cells such as ovarian cancer, breast cancer, and liver cancer [50][41]. It also has anti-inflammatory, bacteriostatic, antiviral, and other functions. The structural modification of rhein to improve its efficacy, solubility, and other physical and chemical properties has become the focus of current research.
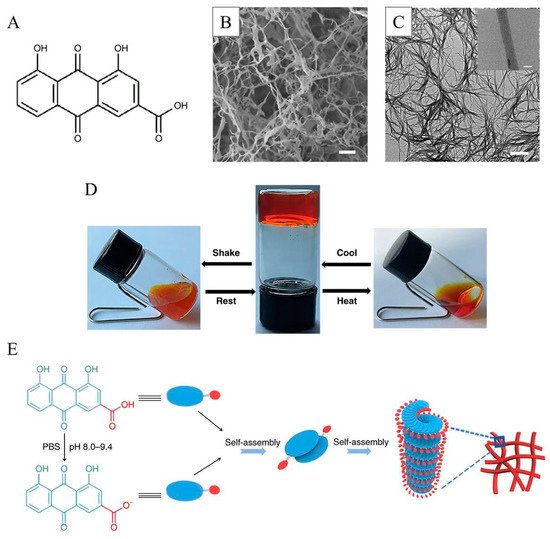
Zheng et al. [51][42] found that rhein could be self-assembled directly into an orange-red nanofiber supramolecular hydrogel with a three-dimensional network structure after a simple heating–cooling process (Figure 4). The hydrogel has certain stimulus responsiveness, and can undergo a reversible sol-gel transition when the temperature and pH value are changed. The gel can release rhein slowly, and it is easier to enter cells and bind to the TLR4 receptor on the cell surface, thereby significantly inhibiting dephosphorylating IκBα and the p65 nuclear metastasis in the NFκB signaling pathway of BV2 microglia induced by lipopolysaccharide, and alleviating neuroinflammatory response for a long time. The authors believed that the π–π stacking between anthraquinones and the hydrogen bonding and the electrostatic interaction between molecules play an important role in the stable formation of this hydrogel. Feng et al. [52][43] also conducted a detailed study on the formation of this rhein-based hydrogel through molecular simulation and considered that the ionization of the carboxyl group of rhein at different pH conditions plays a crucial role in the formation of this gel.

Figure 4. Schematic illustration of the rhein-based hydrogel: (A) Chemical structure of rhein; (B) SEM image of the rhein hydrogel; (C) TEM image of the rhein hydrogel; (D) Reversible sol–gel transitions of rhein-based hydrogel triggered by shear stress and temperature; (E) Self-assembly mechanism of the rhein-based hydrogel. Reproduced with permission from Copyright (2019) Nature Publishing Group.
5. Curcumin-Based Low-Molecular-Weight Supramolecular Gels
Curcumin (Cur), a hydrophobic polyphenol compound extracted from the rhizome of turmeric, has various pharmacological effects such as antioxidant, anti-inflammatory, hypoglycemic, antihyperlipidemic, neuroprotection, and so on. Clinical experiments also demonstrated that curcumin could inhibit the generation, transformation, proliferation, and metastasis of tumor cells, while has little toxicity to normal cells [55][44]. Because of a strong conjugation structure formed by two benzene rings and heptadienedione units, curcumin also has the disadvantage of high molecular rigidity, small water solubility, photothermal sensitivity, and low water stability, which greatly limit its applications in clinical practice.
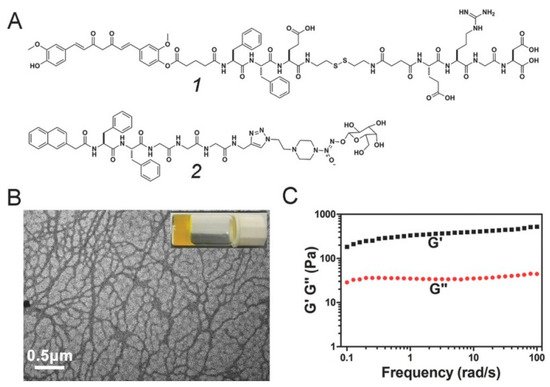
To overcome these shortcomings, Yang et al. [56][45] reported the first curcumin derivative-based supramolecular hydrogel in 2014. Firstly, the short peptide FFE-ss-ERGD containing disulfide bond was coupled with Cur by using glutaric anhydride as a coupling agent to obtain Cur-FFE-ss-ERGD precursor compound (compound 1 in Figure 5), which could be converted into Cur-FFE-SH gelator under the reduction in GSH. The formed Cur-FFE-SH gelators could be transformed into a yellowish hydrogel in situ under physiological conditions. The obtained supramolecular gel has an intertwined filamentous nanofiber structure, which can release Cur slowly due to the hydrolysis of the ester bond in PBS, and is expected to be used in the local treatment of cancer. Moreover, a kind of gel precursor Nap-FFGGG-NO which could produce NO (compound 2 in Figure 5) was mixed with the above Cur-FFE-ss-ERGD precursor compound 1. Under the action of GSH, a mixed supramolecular gel capable of releasing Cur and NO together would be obtained for the treatment of myocardial infarction. Through intramyocardial injection, the combination of curcumin and NO was found to significantly reduce collagen deposition, improve cardiac function, inhibit apoptosis and hypertrophy, and promote poor myocardial remodeling, showing a promise in the treatment of cardiovascular diseases [57][46]. To improve the targeting ability of the curcumin-based hydrogel, the group also introduced glycyrrhetinic acid (GA) into short peptide through amidation reaction and further obtained GA-GFFYK(Cur)E-ss-ERGD (GA-Cur) gel precursor [58][47]. Because GA can specifically bind to the over-expressed GA receptor protein kinase C on the membrane of liver cancer cells, the curcumin gel precursor has active targeting properties and could enter the liver cancer cells through endocytosis. Under the action of the high content of GSH inside the cell, the disulfide bond of the precursor will be broken, and then a hydrogel will be formed in situ by self-assembly. Cur can be slowly released by hydrolysis of ester bonds. The results suggested that the targeted gels with higher cellular uptake and anti-tumor efficacy are promising approaches for the treatment of liver cancer.

Figure 5. Schematic illustration of the curcumin-based mixed component supramolecular hydrogel: (A) Chemical structure of compounds 1 and 2; (B) TEM image and optical image of the mixed component hydrogel; (C) Dynamic frequency sweep of the mixed component hydrogel (strain = 1%). Reproduced with permission from Copyright (2017) Wiley-VCH GmbH.
6. Oleanolic Acid Based Low-Molecular-Weight Supramolecular Gels
Oleanolic acid (OA) is a well-known pentacyclic triterpene with an oleanol skeleton, which exists in many plants in free form or combined into glycosides. At present, it is mainly isolated and extracted from the fruits of Gentianaceae plant Gentiana or Ligustrum lucidum. OA has a curative effect on liver protection and has been used in the clinic for many years. Furthermore, OA has a variety of pharmacological activities, such as hypoglycemic, antioxidant, anticancer, antiviral, anti-platelet aggregation, and so on [62][48].
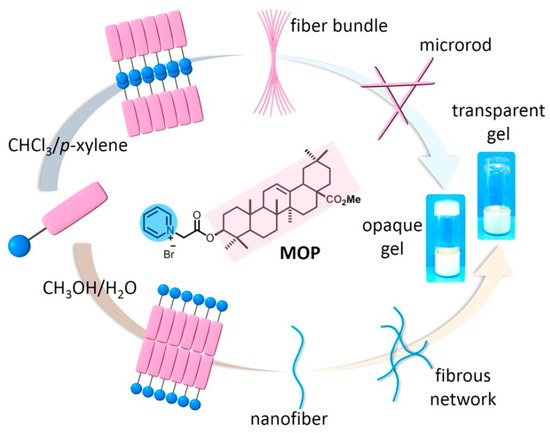
Gao et al. [66][49] further studied in detail the effect of solvent polarity on the assembly of oleanolic acid derivatives (Figure 6). The author first modified the A ring of ethyl oleanolic acid with pyridinium to obtain the amphiphilic derivative MOP and then studied its self-assembly using chloroform/p-xylene or methanol/water mixed solvents. The results showed that, due to the influence of solvation, van der Waals force, and other non-covalent interaction, with the addition of p-xylene in a chloroform/p-xylene solvent system, MOP first assembled into spherical nanoparticles, gradually transformed into microrods, and finally formed an opaque gel. While, in methanol/water solvent, with the addition of water, MOP changed from spherical nanoparticle to nanofiber and finally formed a transparent gel. This research provided a basis for the assembly of natural drugs. Vega-Granados et al. [67][50] also modified the aromatic ring on the carboxyl group of the OA skeleton and then dissolved the resulting derivative in hot organic solvents. Similar to the previous method, the authors found that the derivative can also be quickly assembled into an organogel when cooled in the mixed solvents of DMSO/water or DMF/water. The authors also found that the DMSO or DMF solution of this derivative could spontaneously acquire moisture in the air, and then slowly self-assembled into a gel with a more uniform structure and greater mechanical strength.

Figure 6. Schematic illustration of the solvent-directed assembly of oleanolic-acid-based amphiphilic derivative MOP in chloroform/p-xylene and methanol/water systems, respectively. Reprinted with permission from Copyright (2016) American Chemical Society.
7. Glycyrrhetinic Acid Based Low-Molecular-Weight Supramolecular Gels
As a valuable traditional Chinese medicine, the main active ingredient of Glycyrrhiza uralensis Fisch is glycyrrhetinic acid (GA) and its diglucuronide glycyrrhizic acid [70][51]. Glycyrrhetinic acid, similar to oleanolic acid, also belongs to oleanane-type pentacyclic triterpene, which has a variety of biological activities, such as anticancer, anti-inflammatory, antiviral, antibacterial, and liver protection, and its structure is easy to modify. Therefore, there are an increasing number of related studies in recent years.
Glycyrrhetinic acid has a special five-ring three terpene structure, in which the A ring and E ring have hydroxyl and carboxyl substituents, respectively, and the C ring has an alkene structure, so it may be directly self-assembled into an organogel. In 2012, Bag et al. [71][52] reported the first glycyrrhetinic-acid-based supramolecular organogel. The author found that, after heating and dissolving glycyrrhetinic acid in some specific organic solvents, a translucent gel could be obtained after the cooling process. These solvents include 13 kinds of common aromatic solvents, ethylene glycol, glycerol, aliphatic chlorinated hydrocarbons, and mixed solvents of DMSO/water and DMF/water. The hydrogen bonding between hydroxyl and carboxyl groups in the structure of glycyrrhetinic acid plays an important role in the formation of the gel. The obtained organogel has a flower-like spherical or sphere-like microstructure and can be used as a template for the formation of CdS nanoparticles. The Ju group also modified the hydroxyl group of A-ring of methyl glycyrrhetinate with the pyridine group and obtained a new amphiphilic MGP molecule, which could be self-assembled into spiral nanofibers by π–π stacking interaction, van der Waals interaction, and hydrophilic interaction in chloroform/aromatic solvents [72][53]. This nanofiber can further assemble into a transparent chiral organogel through mutual entanglement. This study provided a simple and effective method for the construction of supramolecular chiral assemblies. The group also prepared an organogel through the charge transfer effect of pyrene modified glycyrrhetinic acid and 2,4,7-trinitrofluorenonefluorine in mixed solvents of DMSO/water or DMF/water [73][54].
8. Betulinic Acid/Betulin-Based Low-Molecular-Weight Supramolecular Gels
Betulinic acid and betulin are lupane pentacyclic triterpenoids extracted from the bark of jujube and birch trees, which have anti-tumor, anti-cancer, anti-diabetes, and other pharmacological activities. The difference in chemical structure between betulinic acid and betulin only lies in the substituent at C-28, which is also the reason why betulinic acid has a higher biological activity than betulin [81][55].
Due to its unique structural characteristics, betulinic acid can be directly assembled to form a gel. It was found that betulinic acid can be assembled to form nanofiber organogels in 19 common aromatic solvents, alcohol solvents, and so on [26]. The low water solubility of betulinic acid hinders the formation of betulinic-acid-based supramolecular hydrogels. To overcome this problem, Bag et al. [82][56] used the more hydrophilic sodium and potassium salts of betulinic acid as the gel building blocks for research. The results showed that two kinds of salts could form opaque organogels in DMSO/water, ethanol/water, and other mixed solvents. At the same time, two kinds of salts could also be directly assembled in water to form nanofiber hydrogels. The obtained hydrogel had good stability and could be sealed for several months at room temperature. The authors believed that the main forces for the formation of the gel were the electrostatic interaction and the dipole–dipole interaction between molecules. This charged hydrogel can adsorb rhodamine B, fluorescein, neutral red, and other colored dyes, and is expected to be applied to wastewater treatment. Moreover, because jujube bark contains phenolic compounds that could reduce Au (III) to Au (0), the author also used jujube bark extract and Au (III) colloid mixed with two kinds of salts, respectively, and in-situ synthesized gold nanoparticles doped by gel hybrid materials.
Betulin can also be directly assembled to form a gel without any structural modification. Bag et al. [83][57] found that betulin could be assembled into opaque gels in o-xylene, m-xylene, p-xylene, DMSO/water, or other solvents by hydrogen bonding. Microstructural studies showed that all the gel self-assemblies had symmetrical flower-like structures with a diameter of nanometers. The gel had a porous microstructure and could absorb and remove toxic dyes such as methylene blue and crystal violet. Besides, the gel can also be used as a biocompatible carrier to deliver doxorubicin and other drugs, which is expected to be used in the drug delivery system.
9. Other Natural-Drugs-Based Low-Molecular-Weight Supramolecular Gels
Ursolic acid (UA) is a pentacyclic triterpenoid compound of ursolic type. Similar to oleanolic acid, it also has a variety of pharmacological activities such as anti-tumor, anti-oxidation, anti-virus, and so on [84][58]. Lu et al. [85][59] found for the first time that ursolic acid can be self-assembled to form an organogel in organic solvents, such as bromobenzene, without any modification. The intermolecular hydrogen bonds and triterpene skeletons play an important role in the formation of gels. Furthermore, the author introduced the aromatic ring into the molecular structure of ursolic acid through the connection of the amide bond. Using the π–π stacking effect of the aromatic ring and the hydrogen bonding effect of the amide bond, ursolic acid can also self-assemble into a transparent nanofiber organogel in other aromatic solvents, such as chlorobenzene, which provided a new idea for the design of supramolecular gels [86][60].
Puerarin is a kind of isoflavone derivative isolated from traditional Chinese medicine Pueraria lobata. It has antipyretic, sedative, and crown-dilating effects, and is mainly used in the treatment of coronary heart disease, angina pectoris, and hypertension [87][61]. Cai et al. [88][62] directly used the self-assembly of puerarin to form a supramolecular gel with excellent oxidation resistance and acid resistance through a heating–cooling operation. The gel could overcome the injury of exogenous reactive oxygen species and improve the survival rate of H2O2-treated cells through the down-regulated activity of superoxide dismutase and the content of malondialdehyde in bone marrow mesenchymal stem cells. The dissociation rate of the puerarin-based gel in simulated intestinal juice was also significantly faster than that in simulated gastric juice, indicating that the puerarin-based gel is expected to be used in oral preparations. However, some studies also found that the obtained gel also has some limitations such as poor mechanical strength and thermal stability [89][63]. Given this, Li et al. [90][64] used N-(9-fluorenylmethoxycarbonyl)-L-phenylalanine (Fmoc-Phe-OH) to blend with puerarin to form a double interpenetrating network structure of Fmoc-Phe-OH nanofibers and puerarin, resulting in an enhanced puerarin-based hydrogel. The mechanical strength and thermal stability of this gel have been greatly improved, and it still has pH responsiveness. At the same time, because it contains Fmoc in the structure, it has antibacterial properties. The gel can load and release the antibacterial model drug berberine hydrochloride, thus playing a synergistic antibacterial effect.
In addition, due to the essential role of carbohydrate compounds in life activities, the low-molecular-weight supramolecular gels based on carbohydrates have also received great attention [91,92][65][66]. For example, Bielejewski et al. [93,94,95][67][68][69] used methyl-4,6-O-(p-nitrobenzylidene)-α-D-glucopyranoside as the gelators and prepared a series of supramolecular gels. They found that this unique saccharide gelators could form hydrogel in water, or organogels in some usual organic solvents by a heating–cooling process. Especially, this gelators could also form a recoverable ionogel with potential applications in electrochemical devices in the ionic liquid tetramethylammonium bromide by self-assembly of hydrogen bonding. Oosumi et al. [96][70] also designed a type of bola-amphiphilic glycolipid-type supramolecular hydrogelators via a one-pot reaction between various 4-aminophenyl monosaccharide derivatives and 2,3-dichloromaleimide derivatives bearing a carboxy group. These monosaccharides included α-D-galactose, β-D-galactose, α-D-glucose, β-D-glucose, and α-D-mannose. These prepared hydrogelators will form hydrogels by self-assembly during a heating–cooling cycle, which could also exhibit color changes along with the gel–sol transition. However, it should be mentioned that, since many small molecular carbohydrates, mainly monosaccharides and disaccharides, are not usually used directly as drugs, there are only a few reports on the application of low-molecular-weight supramolecular gels based on carbohydrates in the medical field, such as wound healing [97][71] and cell culture [98][72].
Besides the natural drugs mentioned above, other supramolecular gels based on coumarin [99][73], anthracene [100][74], arjunolic acid [101][75], poricoic acid A [102][76], and soybean sterol [103][77] also showed great research and application prospects, which attracted the attention of a large number of researchers.
References
- Gu, Y.W.; Zhao, J.L.; Johnson, J.A. Polymer networks: From plastics and gels to porous frameworks. Angew. Chem. Int. Ed. 2020, 59, 5022–5049.
- Du, X.W.; Zhou, J.; Shi, J.F.; Xu, B. Supramolecular hydrogelators and hydrogels: From soft matter to molecular biomaterials. Chem. Rev. 2015, 115, 13165–13307.
- Jian, X.T.; Feng, X.Y.; Luo, Y.N.; Li, F.J.; Tan, J.Y.; Yin, Y.L. Development, preparation and biomedical applications of DNA-based hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 661409.
- Esposito, C.L.; Kirilov, P.; Roullin, V.G. Organogels, promising drug delivery systems: An update of state-of-the-art and recent applications. J. Control. Release 2018, 271, 1–20.
- Zhang, L.; Jiang, D.W.; Dong, T.H.; Das, R.; Pan, D.; Sun, C.Y. Overview of ionogels in flexible electronics. Chem. Rec. 2020, 20, 948–967.
- Eelkema, R.; Pich, A. Pros and cons: Supramolecular or macromolecular: What is best for functional hydrogels with advanced properties? Adv. Mater. 2020, 32, 1906012.
- Draper, E.R.; Adams, D.J. Low-molecular-weight gels: The state of the art. CHEM 2017, 3, 390–410.
- Chivers, P.R.A.; Smith, D.K. Shaping and structuring supramolecular gels. Nat. Rev. Mater. 2019, 4, 463–478.
- Zhang, J.Y.; Zeng, L.H.; Feng, J. Dynamic covalent gels assembled from small molecules: From discrete gelators to dynamic covalent polymers. Chin. Chem. Lett. 2017, 28, 168–183.
- Bhattacharya, S.; Samanta, S.K. Soft-nanocomposites of nanoparticles and nanocarbons with supramolecular and polymer gels and their applications. Chem. Rev. 2016, 116, 11967–12028.
- Yu, X.D.; Chen, L.M.; Zhang, M.M.; Yi, T. Low-molecular-mass gels responding to ultrasound and mechanical stress: Towards self-healing materials. Chem. Soc. Rev. 2014, 43, 5346–5371.
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting-self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251.
- Zhi, K.K.; Xin, Y. Natural product gels and their gelators. Prog. Chem. 2019, 31, 1314.
- Gao, Y.X.; Hu, J.; Ju, Y. Supramolecular self-assembly based on natural small molecules. Acta Chim. Sin. 2016, 74, 312–329.
- Beutler, J.A. Natural products as a foundation for drug discovery. Curr. Protoc. Pharmacol. 2019, 86, e67.
- Li, G.; Lou, H.X. Strategies to diversify natural products for drug discovery. Med. Res. Rev. 2018, 38, 1255–1294.
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541.
- Li, C.; Wang, J.C.; Wang, Y.G.; Gao, H.L.; Wei, G.; Huang, Y.Z. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162.
- Bruneau, M.; Bennici, S.; Brendle, J.; Dutournie, P.; Limousy, L.; Pluchon, S. Systems for stimuli-controlled release: Materials and applications. J. Control. Release 2019, 294, 355–371.
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging frontiers in drug delivery. J. Am. Chem. Soc. 2016, 138, 704–717.
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071.
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603.
- Norouzi, M.; Nazari, B.; Miller, D.W. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov. Today 2016, 21, 1835–1849.
- Acree, W.E.; Bertrand, G.L. A cholesterol-isopropanol gel. Nature 1977, 269, 450.
- Gao, Y.; Kuang, Y.; Guo, Z.F.; Guo, Z.H.; Xu, B. Enzyme-instructed molecular self-assembly confers nanofibers and a supramolecular hydrogel of taxol derivative. J. Am. Chem. Soc. 2009, 131, 13576–13577.
- Bag, B.G.; Dash, S.S. First self-assembly study of betulinic acid, a renewable nano-sized, 6-6-6-6-5 pentacyclic monohydroxy triterpenic acid. Nanoscale 2011, 3, 4564–4566.
- Stage, T.B.; Bergmann, T.K.; Kroetz, D.L. Clinical pharmacokinetics of paclitaxel monotherapy: An updated literature review. Clin. Pharmacokinet. 2018, 57, 7–19.
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Canovas-Diaz, M.; Puente, T.D. A compressive review about taxol®: History and future challenges. Molecules 2020, 25, 5986.
- Mei, B.; Miao, Q.Q.; Liang, G.L. Enzyme-instructed self-assembly of taxol promotes axonal branching. Nanoscale 2015, 7, 15605–15608.
- Ling, Y.; Gao, Y.; Shu, C.; Zhou, Y.; Zhong, W.Y.; Xu, B. Using a peptide segment to covalently conjugate doxorubicin and taxol for the study of drug combination effect. RSC Adv. 2015, 5, 101475–101479.
- Wang, H.M.; Yang, C.H.; Wang, L.; Kong, D.L.; Zhang, Y.J.; Yang, Z.M. Self-assembled nanospheres as a novel delivery system for taxol: A molecular hydrogel with nanosphere morphology. Chem. Commun. 2011, 47, 4439–4441.
- Mao, L.N.; Wang, H.M.; Tan, M.; Ou, L.L.; Kong, D.L.; Yang, Z.M. Conjugation of two complementary anti-cancer drugs confers molecular hydrogels as a co-delivery system. Chem. Commun. 2012, 48, 395–397.
- Shu, C.; Sabi-Mouka, E.M.B.; Yang, W.; Li, Z.Y.; Ding, L. Effects of paclitaxel (PTX) prodrug-based self-assembly peptide hydrogels combined with suberoylanilide hydroxamic acid (SAHA) for PTX-resistant cancer and synergistic antitumor therapy. RSC Adv. 2016, 6, 100765–100771.
- Li, X.L.; Yu, N.; Li, J.; Bai, J.N.; Ding, D.; Tang, Q.Y. A novel “carrier-free” nanofiber co-delivery system with synergistic antitumor effect of paclitaxel and tetrandrine through the enhancement of mitochondria apoptosis. ACS Appl. Mater. Interfaces 2020, 12, 10096–10106.
- Chakroun, R.W.; Wang, F.H.; Lin, R.; Wang, Y.; Su, H.; Pompa, D. Fine-tuning the linear release rate of paclitaxel-bearing supramolecular filament hydrogels through molecular engineering. ACS Nano 2019, 13, 7780–7790.
- Martino, E.; Della Volpe, S.; Terribile, E.; Benetti, E.; Sakaj, M.; Centamore, A. The long story of camptothecin: From traditional medicine to drugs. Bioorg. Med. Chem. Lett. 2016, 27, 701–707.
- Botella, P.; Rivero-Buceta, E. Safe approaches for camptothecin delivery: Structural analogues and nanomedicines. J. Control. Release 2017, 247, 28–54.
- Su, H.; Wang, F.H.; Wang, Y.Z.; Cheetham, A.G.; Cui, H.G. Macrocyclization of a class of camptothecin analogues into tubular supramolecular polymers. J. Am. Chem. Soc. 2019, 141, 17107–17111.
- Wang, F.H.; Su, H.; Lin, R.; Chakroun, R.W.; Monroe, M.K.; Wang, Z.Y. Supramolecular tubustecan hydrogel as chemotherapeutic carrier to improve tumor penetration and local treatment efficacy. ACS Nano 2020, 14, 10083–10094.
- Wang, F.H.; Xu, D.Q.; Su, H.; Zhang, W.J.; Sun, X.R.; Monroe, M.K. Supramolecular prodrug hydrogelator as an immune booster for checkpoint blocker-based immunotherapy. Sci. Adv. 2020, 6, eaaz8985.
- Henamayee, S.; Banik, K.; Sailo, B.L.; Shabnam, B.; Harsha, C.; Srilakshmi, S. Therapeutic emergence of rhein as a potential anticancer drug: A review of its molecular targets and anticancer properties. Molecules 2020, 25, 2278.
- Zheng, J.; Fan, R.; Wu, H.Q.; Yao, H.H.; Yan, Y.J.; Liu, J.M. Directed self-assembly of herbal small molecules into sustained release hydrogels for treating neural inflammation. Nat. Commun. 2019, 10, 1–12.
- Feng, Y.H.; Zhang, X.P.; Hao, Y.Y.; Ren, G.Y. Simulation study of the pH sensitive directed self-assembly of rheins for sustained drug release hydrogel. Colloids Surf. B Biointerfaces 2020, 195, 111260.
- Patel, S.S.; Acharya, A.; Ray, R.S.; Agrawal, R.; Raghuwanshi, R.; Jain, P. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 887–939.
- Yang, C.B.; Wang, Z.Y.; Ou, C.W.; Chen, M.S.; Wang, L.; Yang, Z.M. A supramolecular hydrogelator of curcumin. Chem. Commun. 2014, 50, 9413–9415.
- Chen, G.Q.; Li, J.L.; Song, M.C.; Wu, Z.Y.; Zhang, W.Z.; Wang, Z.Y. A mixed component supramolecular hydrogel to improve mice cardiac function and alleviate ventricular remodeling after acute myocardial infarction. Adv. Funct. Mater. 2017, 27, 1701798.
- Chen, G.Q.; Li, J.L.; Cai, Y.B.; Zhan, J.; Gao, J.; Song, M.C. A glycyrrhetinic acid-modified curcumin supramolecular hydrogel for liver tumor targeting therapy. Sci. Rep. 2017, 7, 44210.
- Lin, C.; Wen, X.A.; Sun, H.B. Oleanolic acid derivatives for pharmaceutical use: A patent review. Expert Opin. Ther. Pat. 2016, 26, 643–655.
- Gao, Y.X.; Hao, J.; Wu, J.D.; Zhang, X.; Hu, J.; Ju, Y. Solvent-directed assembly of a pyridinium-tailored methyl oleanolate amphiphile: Stepwise growth of microrods and nanofibers. Langmuir 2016, 32, 1685.
- Vega-Granados, K.; Ramírez-Rodríguez, G.B.; Contreras-Montoya, R.; Ramirez, F.J.; Palomo, L.; Parra, A. Atmospheric water triggers supramolecular gel formation of novel low molecular weight maslinic and oleanolic triterpenic derivatives. Mater. Chem. Front. 2019, 3, 2637–2646.
- Wang, L.Q.; Yang, R.; Yuan, B.C.; Liu, Y.; Liu, C.S. The antiviral and antimicrobial activities of licorice; a widely-used Chinese herb. Acta Pharm. Sin. B 2015, 5, 310–315.
- Bag, B.G.; Majumdar, R. Self-assembly of a renewable nano-sized triterpenoid 18β-glycyrrhetinic acid. RSC Adv. 2012, 2, 8623–8626.
- Gao, Y.X.; Hao, J.; Wu, J.D.; Zhang, X.; Hu, J.; Ju, Y. Supramolecular helical nanofibers assembled from a pyridinium-functionalized methyl glycyrrhetate amphiphile. Nanoscale 2015, 7, 13568–13575.
- Hu, J.; Wu, J.D.; Wang, Q.; Ju, Y. Charge-transfer interaction mediated organogels from 18β-glycyrrhetinic acid appended pyrene. Beilstein J. Org. Chem. 2013, 9, 2877–2885.
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951.
- Bag, B.G.; Dash, S.S. Self-assembly of sodium and potassium betulinates into hydro- and organo-gels: Entrapment and removal studies of fluorophores and synthesis of gel-gold nanoparticle hybrid materials. RSC Adv. 2016, 6, 17290–17296.
- Bag, B.G.; Dash, S.S. Hierarchical self-assembly of a renewable nanosized pentacyclic dihydroxy-triterpenoid betulin yielding flower-like architectures. Langmuir 2015, 31, 13664–13672.
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic acid-based derivatives as potential anti-cancer agents: An update. Int. J. Mol. Sci. 2020, 21, 5920.
- Lu, J.R.; Wu, X.N.; Liu, L.; Chen, H.P.; Liang, Y.H. First organogelation study of ursolic acid; a natural ursane triterpenoid. Chem. Lett. 2016, 45, 860–862.
- Lu, J.R.; Hu, J.S.; Liang, Y.H.; Cui, W.Q. The supramolecular organogel formed by self-assembly of ursolic acid appended with aromatic rings. Materials 2019, 12, 614.
- Zhou, Y.X.; Zhang, H.; Peng, C. Puerarin: A review of pharmacological effects. Phytother. Res. 2014, 28, 961–975.
- Cai, Y.B.; Zhang, J.W.; He, Y.Y.; Li, Z.H.; Hua, Y.Q.; Wu, Z.Y. A supramolecular hydrogel of puerarin. J. Biomed. Nanotechnol. 2018, 14, 257–266.
- Pang, Z.T.; Wei, Y.F.; Wang, N.N.; Zhang, J.J.; Gao, Y.; Qian, S. Gel formation of puerarin and mechanistic study during its cooling process. Int. J. Pharm. 2018, 548, 625–635.
- Li, W. Supramolecular nanofiber-reinforced puerarin hydrogels as drug carriers with synergistic controlled release and antibacterial properties. J. Mater. Sci. 2020, 55, 6669–6677.
- Datta, S.; Bhattacharya, S. Multifarious facets of sugar-derived molecular gels: Molecular features; mechanisms of self-assembly and emerging applications. Chem. Soc. Rev. 2015, 44, 5596–5637.
- Basu, N.; Chakraborty, A.; Ghosh, R. Carbohydrate derived organogelators and the corresponding functional gels developed in recent time. Gels 2018, 4, 52.
- Bielejewski, M.; Łapiński, A.; Luboradzki, R.; Tritt-Goc, J. Influence of solvent on the thermal stability and organization of self-assembling fibrillar networks in methyl-4, 6-O-(p-nitrobenzylidene)-α-D-glucopyranoside gels. Tetrahedron 2011, 67, 7222–7230.
- Bielejewski, M.; Nowicka, K.; Bielejewska, N.; Tritt-Goc, J. Ionic conductivity and thermal properties of a supramolecular ionogel made from a sugar-based low molecular weight gelator and a quaternary ammonium salt electrolyte solution. J. Electrochem. Soc. 2016, 163, G187–G195.
- Bielejewski, M.; Rachocki, A.; Kaszyska, J.; Tritt-Goc, J. The gelation influence on diffusion and conductivity enhancement effect in renewable ionic gels based on a LMWG. Phys. Chem. Chem. Phys. 2018, 20, 5803–5817.
- Oosumi, R.; Ikeda, M.; Ito, A.; Izumi, M.; Ochi, R. Structural diversification of bola-amphiphilic glycolipid-type supramolecular hydrogelators exhibiting colour changes along with the gel-sol transition. Soft Matter 2020, 16, 7274–7278.
- Yang, Z.M.; Liang, G.L.; Ma, M.L.; Abbah, A.S.; Lu, W.W.; Xu, B. D-glucosamine-based supramolecular hydrogels to improve wound healing. Chem. Commun. 2007, 28, 843–845.
- Wang, W.J.; Wang, H.M.; Ren, C.H.; Wang, J.Y.; Tan, M.; Shen, J. A saccharide-based supramolecular hydrogel for cell culture. Carbohydr. Res. 2011, 346, 1013–1017.
- Pan, S.F.; Luo, S.; Li, S.; Lai, Y.S.; Geng, Y.Y.; He, B. Ultrasound accelerated gelation of novel l-lysine based hydrogelators. Chem. Commun. 2013, 49, 8045–8047.
- Wang, P.Y.; Hu, J.; Yang, S.; Song, B.A.; Wang, Q. Self-assembly of pyridinium-tailored anthracene amphiphiles into supramolecular hydrogels. Chem. Asian J. 2015, 9, 2880–2884.
- Bag, B.G.; Majumdar, R. Vesicular self-assembly of a natural triterpenoid arjunolic acid in aqueous medium: Study of entrapment properties and in situ generation of gel-gold nanoparticle hybrid material. RSC Adv. 2014, 4, 53327–53334.
- Zhi, K.K.; Sun, Y.; Zhao, H.T.; Zhang, C.M.; Ping, H.S.; Yang, X. Self-assembled supramolecular material derived from traditional Chinese medicine: Injectable self-assembled natural product gel for drug delivery with biological activity. Mater. Today Commun. 2020, 6, 101149.
- Bag, B.G.; Barai, A.C. Self-assembly of naturally occurring stigmasterol in liquids yielding a fibrillar network and gel. RSC Adv. 2020, 10, 4755–4762.
More
