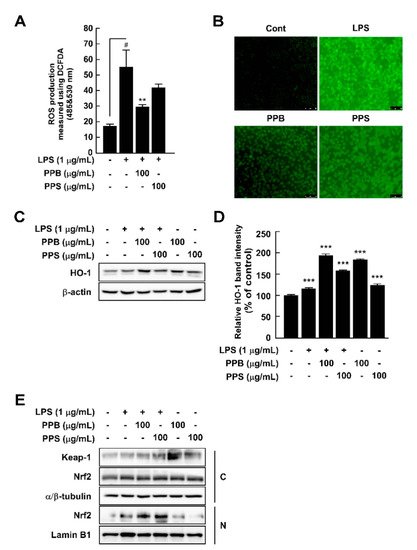
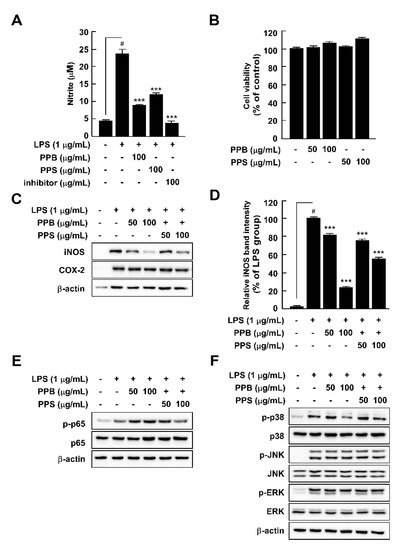
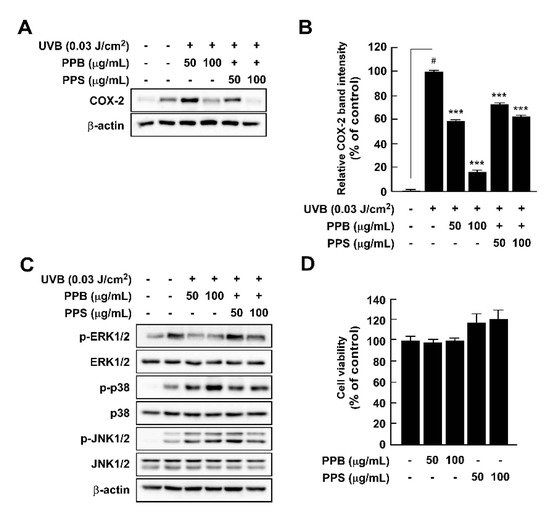
Here, we compared the chemical properties and antioxidant effects of black pepper (Piper nigrum L.) and pink pepper (Schinus molle L.). Additionally, the antioxidant and anti-inflammatory capacities of pink pepper were measured to determine nutraceutical potential. Pink peppers from Brazil (PPB), India (PPI), and Sri Lanka (PPS) had higher Hunter a* (redness) values and lower L* (lightness) and b* (yellowness) values than black pepper from Vietnam (BPV). Fructose and glucose were detected in PPB, PPI, and PPS, but not in BPV. PPB, PPI, and PPS had greater 2,2-diphenyl-1-picrylhydrazyl and 3-ethylbenzothiazoline-6-sulphonic acid radical scavenging stabilities and higher total phenolic contents than BPV. BPV had higher levels of piperine than the pink peppers. Gallic acid, protocatechuic acid, epicatechin, and p-coumaric acid were detected only in the three pink peppers. PPB significantly suppressed lipopolysaccharide-induced reactive oxygen species production with increased Nrf2 translocation from cytosol to nucleus and heme oxygenase-1 expression. PPB and PPS significantly suppressed lipopolysaccharide-induced nitrite production and nitric oxide synthase expression by suppressing phosphorylation of p38 without affecting cell viability. Additionally, PPB and PPS significantly suppressed ultraviolet B-induced cyclooxygenase-2 expression by affecting the phosphorylation of ERK1/2 without cell cytotoxicity. These results suggest that pink pepper is a potential nutraceutical against oxidative and inflammatory stress.
1. Introduction
Reactive oxygen species (ROS), reactive molecules and free radicals derived from molecular oxygen, are responsible for the elimination of microbial invasion
[1]. Although phagocytes, such as macrophages, are innate immune cells that are important for the elimination of pathogens, including viruses, bacteria, and parasites, the abnormal production of ROS causes oxidative stress and consequent cell death. Additionally, inflammation is closely related to ROS levels because unregulated immune response to a pathogen results in overproduction of ROS, which causes damage to the host organ
[2]. Excessive production of nitric oxide (NO) also plays a critical role in inflammation. Thus, continuously high ROS and NO levels lead to chronic inflammation, which can result in the onset of various diseases, including atherosclerosis, rheumatoid arthritis, diabetes, and cancer
[3][4][3,4]. Therefore, detoxification of ROS is a key step preventing inflammation and the subsequent development of chronic inflammatory diseases.
Heme oxygenase-1 (HO-1) is an Nrf2-regulated gene that has important antioxidant, anti-inflammatory, and antiapoptotic effects
[5]. While Nrf2/HO-1 signaling plays a protective role in the regulation of oxidative stress and inflammation, NF-κB, a heterodimer of p65 and p50/p105, plays a central role in ROS- and pathogen-mediated inflammatory signaling cascades. In addition, mitogen-activated protein kinases (MAPKs) are involved in inflammation
[6]. A previous study reported that ultrasonicated seaweed extract suppressed lipopolysaccharide (LPS)-induced nitric oxide synthase (iNOS) expression and nitrite production via regulation of MAPK phosphorylation without affecting the NF-κB signaling cascade in RAW264.7 cells
[7]. Additionally, the phosphorylation of MAPKs is central in skin inflammation response to ultraviolet (UV) light exposure in vitro and in vivo
[8][9][8,9]. Therefore, in addition to NF-kB, MAPKs may be a prime target for anti-inflammatory nutraceuticals.
Schinus molle L., also known as Peruvian pepper, Brazilian pepper, American pepper, Californian pepper, or molle de Peru, is a fast-growing evergreen tree
[10]. Pink pepper is classified into the Californian/Peruvian type (
S. molle) and the Brazilian type (
S. terebinthifolius)
[11]. Although it is unrelated to the black pepper (
Piper nigrum), the pink fruits of
S. molle are known as pink peppercorns and are used as alternatives to black pepper due to their flavor and pungency
[12]. Research on pink pepper has primarily focused on the chemical composition and physicochemical characteristics of its essential oil and its antimicrobial activities
[13][14][13,14]. The results of recent studies have revealed that polysaccharides from
S. molle fruit have in vitro antioxidant, antigenotoxic, antidiabetic, and antihemolytic effects, as well as in vivo anti-inflammatory and antinociceptive properties
[15]. However, the differences in the chemical compositions and the antioxidant and anti-inflammatory effects of
S. molle fruits from different countries remain unclear.
2. Results and Discussion
2.1. Color and Sugar Content Analysis
Although
S. molle is a known as American pepper, it has distinctive color and chemical compositions compared to black pepper. While the chemical and physiological properties of black pepper have been well studied
[16][17][20,21], the chemical composition and physiological activity of
S. molle are mainly unknown. Therefore, we compared the in vitro antioxidant effect of pink peppers from three different countries and black pepper as well as analyzed the chemical compositions of
S. molle fruits. To the best of our knowledge, this is the first report of this approach and these findings. Pink and black peppers have distinctive colors and shapes. Pictures of the dried pink peppers from three countries and the BPV used in this experiment are shown in
Figure 1.
Figure 1. Photographs of dried pink peppers from different countries and black pepper.
To compare the general characteristics of pink and black peppers, we analyzed the color and carbohydrate contents of the peppers.
Table 1 summarizes the Hunter L, a, b values and fructose and glucose contents of PPB, PPI, and PPS. We determined fructose, glucose, sucrose, and maltose content in the pink and black peppers (S1) using HPLC assay
[18][16]. While fructose and glucose were not detected in BPV, the pink peppers showed relatively high levels of both monosaccharides. PPS and PPB had the highest fructose content (11,829.82 ± 23.73 mg/100 g) and glucose content (9816.07 ± 36.51 mg/100 g). In the HPLC chromatogram of sugar analysis, we detected two peaks that were consistent with the fructose and glucose standards (
Figure S1). Feriani et al. identified fucose (10.90% ± 0.024%) in
S. molle fruits from Tunisia, and interestingly, glucose was not detected
[15]. On the HPLC chromatogram obtained for pink pepper, we confirmed two peaks that were consistent with the standard peaks of fructose and glucose, but no peaks corresponding to fucose were detected (
Figure S1). Therefore, we ruled out the hypothesis that fucose would be present in considerable amount in commercial
S. molle. Further, Solis et al. reported that fresh and residue seeds of
S. molle contained 16% glucose; however, they did not confirm fructose and fucose
[19][22]. In our method, we used 70% EtOH for sample extraction, while Feriani et al. used hot water extraction for
S. molle fruits from Tunisia. Therefore, these differences in monosaccharide contents seem to be due to the differences in the origin of the peppers, cultivation environment, and the extraction method.
Table 1. Color and sugar composition of pink and black peppers.
| |
Region |
L |
a |
b |
Fructose
(mg/100 g) |
Glucose
(mg/100 g) |
| Pink |
Brazil |
28.87 ± 0.01 b |
11.94 ± 0.01 a |
11.41 ± 0.004 b |
11,507.21 ± 90.5 b |
9816.07 ± 36.51 a |
| India |
25.02 ± 0.09 d |
6.71 ± 0.05 b |
8.55 ± 0.04 d |
9528.74 ± 46.67 c |
6181.37 ± 315.61 b |
| Sri Lanka |
27.36 ± 0.03 c |
11.92 ± 0.06 a |
10.89 ± 0.05 c |
11,829.82 ± 23.73 a |
9758.15 ± 330.28 a |
| Black |
Vietnam |
43.65 ± 0.01 a |
3.81 ± 0.02 c |
13.16 ± 0.01 a |
N.D. |
N.D. |