During the past two decades, immobilization of titanium dioxide (TiO2), a well-known photocatalyst, on several polymeric substrates has extensively gained ground since it limits the need of post-treatment separation stages. Taking into account the numerous substrates tested for supporting TiO2 photocatalysts, the use of biodegradable polymer seems a hopeful option owing to its considerable merits, including the flexible nature, low price, chemical inertness, mechanical stability and wide feasibility.
- biodegradable polymers
- titanium dioxide
- immobilization
- photocatalysis
- wastewater remediation
1. Introduction
Water contamination by organic compounds and metals has been outlined as one of the major global problems nowadays. In fact, due to their non-biodegradable nature, these harmful compounds remain for long time periods after their discharge into the environment, thus being characterized as persistent contaminants. Several techniques have been explored to remove these pollutants from water, including adsorption and photocatalysis, which comprise attractive and eco-friendly approaches. The nano-sized titanium dioxide (TiO2) is a famous photocatalyst among the metal oxides, due to its excellent efficiency, low price, physicochemical stability, extensive disposal, safety, and non-corrosive behavior. It has three crystal forms, anatase, rutile and brookite, while the first presents the most effective photocatalytic performance. Nevertheless, due to the challenges that arise from the very small particle size and the unfeasible reusability of the particles, including the post separation and recovery of the photocatalytic particles after water or wastewater treatment, the need of TiO2 immobilization is crucial [1] (Figure 1).
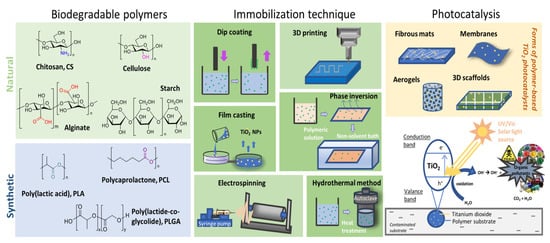
2. Biodegradable Polymers Combined with TiO2 for Enhanced Photocatalytic Activity
2.1. Natural Biodegradable Polymers
2.1.1. Chitosan (CS)
Synthetic and Characterization Routes
2.1. Natural Biodegradable Polymers
2.1.1. Chitosan (CS)
Synthetic and Characterization Routes
A biodegradable polymer that has been widely explored in green pathways for waste remediation and photocatalytic activities is chitosan (CS). It is a linear polysaccharide and one of the most abundant biopolymers in the nature, with biodegradable, biocompatible and non-toxic character, derived from the deacetylation process of chitin, found in the exoskeletons of crustaceans and arthropods. Enzymes, such as chitosanase or lysozymes, are known to degrade chitosan. Its low-cost and the several versatile properties that chitosan possesses, render this polymer as an ideal candidate for environmental remediation purposes. Since CS is a great supporting material for the dispersion of TiO2 nanoparticles, CS/TiO2 is one of the most investigated composite photocatalysts, while their synergistic effects between them have also been studied. The immobilization of TiO2 in CS films has been widely investigated since it can be easily obtained owing to the miscibility between CS and hydrophilic TiO2. Chitosan contains in its structure amino and hydroxyl functional groups which act as coordination sites to form complexes with metals and several compounds, boosting by this means the effective removal of pollutants with special selectivity. However, the immobilization attempts require strong affinity between the TiO2 and the substrate, and thus, cross-linking processes with the aid of several alkaline agents (e.g., NaOH) are often selected [15][2]. A brief description of the studies reported herein for CS-supported photocatalysts is presented in Table 1.
| Biodegradable Polymeric Matrix | Photocatalysis Parameters. | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Polymer Substrate | TiO2 Precursor | Dopant | Immobilization Technique | Morphology of the Photocatalyst | Type of (Target) Pollutant | Light Source | Degradation Efficiency (Time Required) | |
| 1 | CS | TiO2 nanopowders (Aeroxide; 80% anatase) | MT | CS-MT film casting & dip-coating in TiO2 formulation | Bilayer photocatalyst | Μethyl orange dye | 45 W fluorescent lamp | 98.7% | [23] |
| 2 | CS-grafted poly(vinyl imidazole) | Titanium isopropoxide | CDs | In situ deposition of TiO2 NPs and CDs onto the polymeric surface under microwave irradiation | Nanocomposite hydrogel | 2,4-dicholorophenol Reactive Blue 4 Reactive Red 15 |
Sunlight exposure for 30 min | 95% (180 min) 95.8% (30 min) 98.2% (30 min) |
[20] |
| 3 | CS | TiO2 (P25) | - | Immobilization of TiO2 in CS film by cross-linking process | Film | Tetracycline hydrochloride | UV lamp 30 W and λ = 360 nm | 87% (240 min) | [15] |
| 4 | CS | Aeroxide P25-TiO2 | - | 3D printing | 3D printed scaffolds | Amoxicillin | UV irradiation (125 W), λ = 300–800 nm | 90–60% (180 min) | [14] |
| 5 | CS | TiO2 powder (P25) | - | One-step spray-drying synthesis | CS/TiO2 nanocomposite particles | Organic dye, crystal violet | RPR-200 Photochemical Reactor (Rayonet), λ = 300 nm (8×, 21 W), and λ = 350 nm (8×, 24 W) lamps | 58.3–15.5% (120 min) 95.7% pristine particles |
[25] |
| 6 | CS | TiO2 | GO | Dopped-GO and CS impregnated in TiO2 solution | - | cefixime trihydrate | 4 × lamps UV-A irradiation, λ = 365 nm | 95.34% (60 min) | [21] |
| 7 | CMCS | Butyl titanate | TiO2/ZrO2 composites | ZrO2:TiO2 were synthesized by a microwave hydrothermal method, CMCS as template | Composites | Rhodamine B | Photochemical reactor-UV irradiation (CEL-LPH120), | 90.5–60.6% (60 min) | [18] |
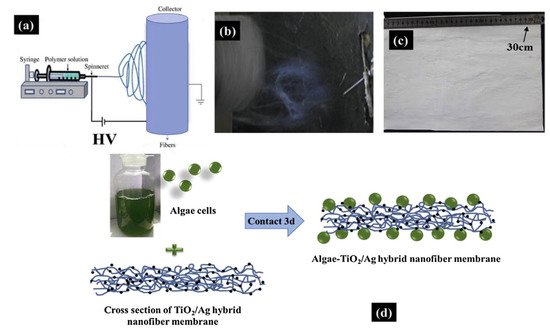
| 8 | CS + PVA | TiO2 (anatase) | Ag | Loading algae cells on the TiO2/Ag CS hybrid nanofiber mat prepared by electrospinning | Algae-TiO2/Ag hybrid nanofiber membrane | Cr(VI) removal | 500 W halogen tungsten lamp, λ > 400 nm | 91–25% (180 min) | [24] |
| 9 | CS + CA | TiO2 nanoparticles | SWCNTs + Fe3O4 | Incorporated inorganics into electrospun nanofibers | Composite nanofibers | Cr(VI), As(V), Methylene blue and Congo red | 4 × UV lamps, 30 W and λ = 365 nm | ~99% (40–60 min) | [22] |
| Biodegradable Polymeric Matrix | Photocatalysis Parameters. | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Polymer Substrate | TiO2 Precursor | Dopant | Immobilization Technique | Morphology of the Photocatalyst | Type of (Target) Pollutant | Light Source | Degradation Efficiency (Time Required) | |
| 1 | CS | TiO2 nanopowders (Aeroxide; 80% anatase) | MT | CS-MT film casting & dip-coating in TiO2 formulation | Bilayer photocatalyst | Μethyl orange dye | 45 W fluorescent lamp | 98.7% | [3] |
| 2 | CS-grafted poly(vinyl imidazole) | Titanium isopropoxide | CDs | In situ deposition of TiO2 NPs and CDs onto the polymeric surface under microwave irradiation | Nanocomposite hydrogel | 2,4-dicholorophenol Reactive Blue 4 Reactive Red 15 |
Sunlight exposure for 30 min | 95% (180 min) 95.8% (30 min) 98.2% (30 min) |
[4] |
| 3 | CS | TiO2 (P25) | - | Immobilization of TiO2 in CS film by cross-linking process | Film | Tetracycline hydrochloride | UV lamp 30 W and λ = 360 nm | 87% (240 min) | [2] |
| 4 | CS | Aeroxide P25-TiO2 | - | 3D printing | 3D printed scaffolds | Amoxicillin | UV irradiation (125 W), λ = 300–800 nm | 90–60% (180 min) | [5] |
| 5 | CS | TiO2 powder (P25) | - | One-step spray-drying synthesis | CS/TiO2 nanocomposite particles | Organic dye, crystal violet | RPR-200 Photochemical Reactor (Rayonet), λ = 300 nm (8×, 21 W), and λ = 350 nm (8×, 24 W) lamps | 58.3–15.5% (120 min) 95.7% pristine particles |
[6] |
| 6 | CS | TiO2 | GO | Dopped-GO and CS impregnated in TiO2 solution | - | cefixime trihydrate | 4 × lamps UV-A irradiation, λ = 365 nm | 95.34% (60 min) | [7] |
| 7 | CMCS | Butyl titanate | TiO2/ZrO2 composites | ZrO2:TiO2 were synthesized by a microwave hydrothermal method, CMCS as template | Composites | Rhodamine B | Photochemical reactor-UV irradiation (CEL-LPH120), | 90.5–60.6% (60 min) | [8] |
| 8 | CS + PVA | TiO2 (anatase) | Ag | Loading algae cells on the TiO2/Ag CS hybrid nanofiber mat prepared by electrospinning | Algae-TiO2/Ag hybrid nanofiber membrane | Cr(VI) removal | 500 W halogen tungsten lamp, λ > 400 nm | 91–25% (180 min) | [9] |
| 9 | CS + CA | TiO2 nanoparticles | SWCNTs + Fe3O4 | Incorporated inorganics into electrospun nanofibers | Composite nanofibers | Cr(VI), As(V), Methylene blue and Congo red | 4 × UV lamps, 30 W and λ = 365 nm | ~99% (40–60 min) | [10] |
Photocatalytic Performance
Photocatalytic Performance
Chitosan based composites achieved a wide range of degradation efficiency reaching 90% in most of the cases, while UV radiation was most frequently employed for the degradation of pollutants. Different structures of chitosan-based materials were used in photocatalytic experiments, such as films, bilayers, 3D printed scaffolds and fibers. Another important factor is the reusability of polymeric materials used in photocatalysis. Chitosan based catalysts were applied for 3–6 cycles in the majority of studies with sufficient results. However, a decrease in the photocatalytic activity was recorded after the second cycle in most of studies. Nevertheless, Bahrudin et al. investigated the photodegradation of methyl orange (MO), in which the reusability of TiO2/CS-Mt composite was tested for 10 cycles. According to this study, two chitosan bilayers were manufactured with or without the addition of Mt. The insertion of Mt showed a favorable effect in charge separation of TiO2. Thus, these bilayers performed a higher photocatalytic performance in degradation of MO dye.
2.1.2. Cellulose
Synthetic and Characterization Routes
2.1.2. Cellulose
Synthetic and Characterization Routes
Cellulose is another non-toxic, biocompatible, as well as biodegradable polysaccharide found in great abundance in nature. It comprises a linear chain with multiple hydroxyl groups able to form hydrogen bonds with other oxygen atoms on the nearby polymeric chain. Biodegradation of cellulose is achieved either by enzymatic oxidation, with peroxidase emitted by fungi, or by bacteria. Cellulose and its derivatives, such as carboxymethyl cellulose, cellulose phosphate, and acetate, have been used primarily as reinforcement materials owing to its excellent mechanical, chemical, and biological properties for dyes and metal binding for environmental remediation purposes.
Photocatalytic Performance
Photocatalytic Performance
Getting an insight into bibliography about the use of cellulose destined for the fabrication of photocatalytic membranes, it was shown that various pollutants were targeted for the examination of their possible removal. The majority of studies investigated the removal of dyes such as MB, RhB, MO, crystal violet, reactive brilliant red K-2BR and cationic red X-GRL. Their degradation was more than 90% in most of the cases and the experiments were carried out under UV light, sunlight, and visible light while the irradiation time ranged from 10 to 180 min [4,9,10,26,27,28,29,30,31,32][12][13][14][11][15][16][17][18][19][20]. Furthermore, other organic pollutants were explored with worth noticing results. Phenol, benzene, toluene, ethylbenzene and xylene were studied under UV and visible light irradiation, while their degradation efficiency ranged between 70% and 90% [28][16], [27][15] after 180–360 min of treatment. Moreover, other toxic pollutants were examined such as stearic acid, Cr(VI), and cyanotoxins microcystin-LR or cylindrospermopsin with remarkable photocatalytic results [9,10,30][13][14][18]. Concerning the photocatalytic substrates, various matrices of cellulose material were tested such as membranes, films, fibers, and magnetic macrospheres. Regarding the reusability of cellulose material in photocatalysis, the synthesized composites were tested for three to six repetition cycles, and as a result the photocatalytic materials showed exhibiting good stability.
2.1.3. Alginate (Alg)
Synthetic and Characterization Routes
2.1.3. Alginate (Alg)
Synthetic and Characterization Routes
Alginate is a natural and anionic polysaccharide, mainly derived from the cell walls of algae. In recent years, the low price and abundance in nature, have rendered the alginate as a polymer of much attention. Due to the presence of hydroxyl and carboxyl groups on its molecule, alginate has been as well explored for the effective removal of metal ions.
Gelation techniques are commonly chosen for the preparation of alginate-based photocatalysts. Within an innovative effort, Dalponte et al. [33][21] reported on the synthesis of a buoyant alginate photocatalyst with immobilized TiO2 nanoparticles for accomplishing both superior photocatalytic behavior and more feasible separation after treatment processes. The ionotropic gelation technique was employed, with CaCO3 or NaHCO3 gas-forming agents added firstly to a TiO2 dispersion with the addition of a sodium alginate solution (Figure 64).

Photocatalytic Performance
Photocatalytic Performance
Getting an insight into bibliography of alginate-based materials with combination of TiO2 nanoparticles, it is observed that they were used for various applications, such as the photo-degradation of common dyes (MO, basic blue 41, tartrazine, RhB), removal of pharmaceuticals (ibuprofen, and sulfamethoxazole), and other organic compounds (2-naphthol). Moreover, alginate-TiO2 nanocomposites were used in different morphologies including fibers, membranes, hydrogel spheres, and papers. The irradiation was carried out under two main sources, UV light or sunlight for a time range between 45–340 min. Photocatalytic materials based on alginate showed a high recyclability with a range of 3–7 cycles [16,33,34,35,36,37,38][22][21][23][24][25][26][27].
2.1.4. Starch
Synthetic and Characterization Routes
2.1.4. Starch
Synthetic and Characterization Routes
Starch is a renewable material with biocompatible and biodegradable nature, low cost and high abundance, and due to this positive profile, it has been widely explored in food, textile, packaging as well as pharmaceutical industries. It mainly consists of amylose and amylopectin, while one of the most challenging chapters concerning its use is its dissolution since the strong inter- and intra-molecular hydrogen bonds and its semicrystalline nature with double helices, require strong polar systems. Biodegradation of starch can mainly proceed via hydrolysis at the acetal bonds by enzymes.
Photocatalytic Performance
2.2. Synthetic Polymers with Biodegradable Nature
2.2.1. Poly(Lactic Acid) (PLA)
Synthetic and Characterization Routes
Photocatalytic Performance
Regarding the literature of starch-based TiO2 materials for the removal of different pollutants from wastewater through photocatalytic process, it was noticed that researchers mainly focused on MB and rhodamine B (RhB) dyes removal, widely used in textile industry. In most studies UV light and/or sunlight radiation was employed for the degradation of different initial concentration of dyes, ranging from 4 to 50 ppm [28][29][30][31][32]. The removal of contaminants ranged within 94–100% in most of the cases, and treated solutions were exposed to irradiation for 90 to 360 min.2.2. Synthetic Polymers with Biodegradable Nature
2.2.1. Poly(Lactic Acid) (PLA)
Synthetic and Characterization Routes
PLA is one of the most dynamic and promising biodegradable synthetic polymers derived from renewable resources, such as corns, sugar beets, wheat and other starch-based products. It can be synthesized mainly by the polycondensation of lactic acid or by ring opening polymerization of lactide with the aid of a catalyst. PLA is totally degraded under compost conditions. Except its wide use in pharmaceutical technology, the fabrication of PLA for the synthesis of TiO2-immobilized photocatalytic materials has limited literature, which is presented below, in brief. The synthetic pathways for these photocatalysts include mainly casting, electrospinning and spin-coating methods.
Photocatalytic Performance
2.2.2. Polycaprolactone (PCL)
Synthetic and Characterization Routes
Photocatalytic Performance
According to the available bibliography, PCL-TiO2 composites were applied for the degradation of organic dyes including MB, RhB, and Reactive Black 5 or even for disinfection from bacteria often found in wastewater effluent, such as E. Coli, C. albicans, and Staphylococcus aureus. The PCL composites were manufactured in different structures for photocatalytic purposes, including nanofibers and membranes. Most experiments were carried out under UV light radiation with treatment time ranging from 80–300 min according to the targeted pollutants and the photocatalytic material used. Moreover, the reusability of materials was monitored up to three cycles exhibiting satisfactory results [5,11,12,46,47,48].2.2.3. Other Synthetic Polymers
There are also few biodegradable polymers which have been explored to prepare photocatalysts for TiO2 immobilization, but the relative reports are limited (Photocatalytic Performance
In the field of bio-based polymers, PLA has also been used for the fabrication of TiO2 composite materials with photocatalytic activity. Three interesting studies used the synthesized composites for the degradation of pharmaceuticals (mainly antibiotics) [33][34][35], while another group of studies examined the degradation of common dyes such as MB and methylene orange [36][37][38]. The polymeric materials, acting as supports for the photocatalysts, were mainly manufactured in two morphologies: films and nanofibers. The degradation efficiency of pollutants ranged within different levels. The degradation of pharmaceuticals was near to 90% in most of cases, while the photocatalytic degradation of the azo dyes varied mostly between 70% and 90%. The photocatalytic experiments were carried out under UV and sunlight irradiation, while an adsorption step was priorly applied. A wide range of irradiation times was applied starting from 2 to 10 h.2.2.2. Polycaprolactone (PCL)
Synthetic and Characterization Routes
PCL (polycaprolactone) is a semi-crystalline aliphatic polyester produced by a ring-opening polymerization of ε-caprolactone, mainly in the presence of tin octanoate as catalyst. It is soluble in many organic solvents, while it possesses a semi-rigid nature in room temperature conditions. Enzymes and fungi simply degrade PCL, while for the enhancement of its biodegradation several copolymers with lactide or glycolide are proposed. Due to its low melting point, advanced rheological and viscoelastic characteristics, PCL has been extensively explored in the manufacture of electrospun porous fibers. Only recently PCL was reported as a template to prepare PCL/TiO2 fibrous mats as dynamic candidates for efficient photocatalysts and thus further exploration in this field should be investigated.Photocatalytic Performance
According to the available bibliography, PCL-TiO2 composites were applied for the degradation of organic dyes including MB, RhB, and Reactive Black 5 or even for disinfection from bacteria often found in wastewater effluent, such as E. Coli, C. albicans, and Staphylococcus aureus. The PCL composites were manufactured in different structures for photocatalytic purposes, including nanofibers and membranes. Most experiments were carried out under UV light radiation with treatment time ranging from 80–300 min according to the targeted pollutants and the photocatalytic material used. Moreover, the reusability of materials was monitored up to three cycles exhibiting satisfactory results [39][40][41][42][43][44].2.2.3. Other Synthetic Polymers
There are also few biodegradable polymers which have been explored to prepare photocatalysts for TiO2 immobilization, but the relative reports are limited (| Biodegradable Polymeric Matrix | Photocatalysis Parameters | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Polymer Substrate | TiO2 Precursor | Dopant | Immobilization technique | Morphology of the Photocatalyst | Type of (Target) Pollutant | Light Source | Degradation Efficiency (Time Required) | |
| 1 |
| PLA + PBAT + PBS |
| Titanium isopropoxide (97 wt%) |
| - |
| - Sol-gel method for theTiO2 nanoparticles |
| - blown film technique | Composite films | Toluene | Photocatalytic oxidation reactor with UV-C lamp 6 W and λ = 254 nm | 52% (270 min) | [ | 7 | ] | [ | 45] | |
| 2 | PLGA | TiO2 nanopowder | - | Air-liquid foaming technique | Porous 3D-PCL scaffolds | Methylene blue E. Coli |
UV lamp light with wavelength 365 nm | 90% (180 min) ~99% (24 h) |
[49] | [46] |
| 3 | PHB & CS oligomers | Titanium (IV) oxide (nano- TiO2) (99.7% anatase nanopowder) | - | Electrospinning/Electrospraying & Impregnation techniques | Hybrid fibrous materials | Methylene Blue Escherichia Coli |
UV light (UVASPOT 400/T, Dr. Honle AG; UV lamp UV 400 F/2; 400 W) | >92% (180 min) 100% (30–60 min) |
[13] | [47] |
3. Conclusions
References
- Malesic-Eleftheriadou, N.; Evgenidou, E.; Kyzas, G.Z.; Bikiaris, D.N.; Lambropoulou, D.A. Removal of antibiotics in aqueous media by using new synthesized bio-based poly(ethylene terephthalate)-TiO2 photocatalysts. Chemosphere 2019, 234, 746–755.
- Ikhlef-Taguelmimt, T.; Hamiche, A.; Yahiaoui, I.; Bendellali, T.; Lebik-Elhadi, H.; Ait-Amar, H.; Aissani-Benissad, F. Tetracycline hydrochloride degradation by heterogeneous photocatalysis using TiO2(P25) immobilized in biopolymer (chitosan) under UV irradiation. Water Sci. Technol. 2020, 82, 1570–1578.
- Bahrudin, N.N.; Nawi, M.A.; Zainal, Z. Insight into the synergistic photocatalytic-adsorptive removal of methyl orange dye using TiO2/chitosan based photocatalyst. Int. J. Biol. Macromol. 2020, 165, 2462–2474.
- Midya, L.; Sarkar, A.N.; Das, R.; Maity, A.; Pal, S. Crosslinked chitosan embedded TiO2 NPs and carbon dots-based nanocomposite: An excellent photocatalyst under sunlight irradiation. Int. J. Biol. Macromol. 2020, 164, 3676–3686.
- Bergamonti, L.; Bergonzi, C.; Graiff, C.; Lottici, P.P.; Bettini, R.; Elviri, L. 3D printed chitosan scaffolds: A new TiO2 support for the photocatalytic degradation of amoxicillin in water. Water Res. 2019, 163, 114841.
- Morlando, A.; Sencadas, V.; Cardillo, D.; Konstantinov, K. Suppression of the photocatalytic activity of TiO2 nanoparticles encapsulated by chitosan through a spray-drying method with potential for use in sunblocking applications. Powder Technol. 2018, 329, 252–259.
- Erim, B.; Ciğeroğlu, Z.; Bayramoğlu, M. Green synthesis of TiO2/GO/chitosan by using leaf extract of Olea europaea as a highly efficient photocatalyst for the degradation of cefixime trihydrate under UV-A radiation exposure: An optimization study with D-optimal design. J. Mol. Struct. 2021, 1234.
- Tian, J.; Shao, Q.; Zhao, J.R.B.; Pan, D.; Dong, M.; Jia, C.; Ding, T.; Wu, T.; Guo, Z. Microwave solvothermal carboxymethyl chitosan templated synthesis of TiO 2 /ZrO 2 composites toward enhanced photocatalytic degradation of Rhodamine B. J. Colloid Interface Sci. 2019, 541, 18–29.
- Wang, L.; Zhang, C.; Gao, F.; Mailhot, G.; Pan, G. Algae decorated TiO2/Ag hybrid nanofiber membrane with enhanced photocatalytic activity for Cr(VI) removal under visible light. Chem. Eng. J. 2017, 314, 622–630.
- ZabihiSahebi, A.; Koushkbaghi, S.; Pishnamazi, M.; Askari, A.; Khosravi, R.; Irani, M. Synthesis of cellulose acetate/chitosan/SWCNT/Fe3O4/TiO2 composite nanofibers for the removal of Cr(VI), As(V), Methylene blue and Congo red from aqueous solutions. Int. J. Biol. Macromol. 2019, 140, 1296–1304.
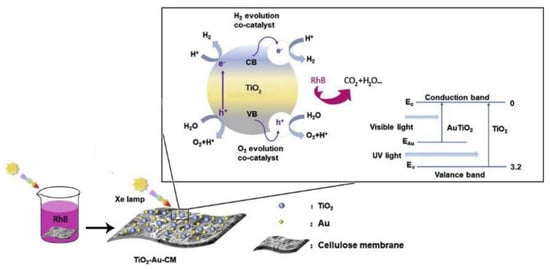
- Yu, Y.; Zhu, X.; Wang, L.; Wu, F.; Liu, S.; Chang, C.; Luo, X. A Simple Strategy to Design 3-Layered Au-TiO2 Dual Nanoparticles Immobilized Cellulose Membranes with Enhanced Photocatalytic Activity. Carbohydr. Polym. 2020, 231.
- Farshchi, E.; Pirsa, S.; Roufegarinejad, L.; Alizadeh, M.; Rezazad, M. Photocatalytic/biodegradable film based on carboxymethyl cellulose, modified by gelatin and TiO2-Ag nanoparticles. Carbohydr. Polym. 2019, 216, 189–196.
- Pinho, L.X.; Azevedo, J.; Miranda, S.M.; Ângelo, J.; Mendes, A.; Vilar, V.J.P.; Vasconcelos, V.; Boaventura, R.A.R. Oxidation of microcystin-LR and cylindrospermopsin by heterogeneous photocatalysis using a tubular photoreactor packed with different TiO2 coated supports. Chem. Eng. J. 2015, 266, 100–111.
- Li, M.; Qiu, J.; Xu, J.; Yao, J. Cellulose/TiO2-Based Carbonaceous Composite Film and Aerogel for Highly Efficient Photocatalysis under Visible Light. Ind. Eng. Chem. Res. 2020, 59, 13997–14003.
- Mohamed, M.A.; Salleh, W.W.N.; Jaafar, J.; Ismail, A.F.; Mutalib, M.A.; Sani, N.A.A.; Asri, S.E.A.M.; Ong, C.S. Physicochemical characteristic of regenerated cellulose/N-doped TiO2 nanocomposite membrane fabricated from recycled newspaper with photocatalytic activity under UV and visible light irradiation. Chem. Eng. J. 2016, 284, 202–215.
- Taghizadeh, M.; Yousefi Kebria, D.; Qaderi, F. Effect of biosurfactant as a novel draw solution on photocatalytic treatment and desalination of produced water by different forward osmosis membranes. Water Sci. Technol. Water Supply 2020, 20, 240–250.
- Lin, Z.; Lu, Y.; Huang, J. A hierarchical Ag2O-nanoparticle/TiO2-nanotube composite derived from natural cellulose substance with enhanced photocatalytic performance. Cellulose 2019, 26, 6683–6700.
- Ullah, S.; Acuña, J.J.S.; Pasa, A.A.; Bilmes, S.A.; Vela, M.E.; Benitez, G.; Rodrigues-Filho, U.P. Photoactive layer-by-layer films of cellulose phosphate and titanium dioxide containing phosphotungstic acid. Appl. Surf. Sci. 2013, 277, 111–120.
- Wittmar, A.S.M.; Fu, Q.; Ulbricht, M. Photocatalytic and magnetic porous cellulose macrospheres for water purification. Cellulose 2019, 26, 4563–4578.
- Li, Y.; Pan, Y.; Zhang, B.; Liu, R. Adsorption and photocatalytic activity of Cu-doped cellulose nanofibers/nano-titanium dioxide for different types of dyes. Water Sci. Technol. 2020, 82, 1665–1675.
- Lan, Y.; Yu, M.; He, D.; Wang, Y.; Meng, Q.B.; Huang, H.; Zhang, Y.; Ma, T.; Song, X.M. Pickering emulsion-embedded hierarchical solid-liquid hydrogel spheres for static and flow photocatalysis. J. Colloid Interface Sci. 2021, 589, 587–596.
- Nouri, L.; Hemidouche, S.; Boudjemaa, A.; Kaouah, F.; Sadaoui, Z.; Bachari, K. Elaboration and characterization of photobiocomposite beads, based on titanium (IV) oxide and sodium alginate biopolymer, for basic blue 41 adsorption/photocatalytic degradation. Int. J. Biol. Macromol. 2020, 151, 66–84.
- Nawaz, M.; Khan, A.A.; Hussain, A.; Jang, J.; Jung, H.Y.; Lee, D.S. Reduced graphene oxide−TiO2/sodium alginate 3-dimensional structure aerogel for enhanced photocatalytic degradation of ibuprofen and sulfamethoxazole. Chemosphere 2020, 261, 127702.
- Dalponte, I.; de Sousa, B.C.; Mathias, A.L.; Jorge, R.M.M. Formulation and optimization of a novel TiO2/calcium alginate floating photocatalyst. Int. J. Biol. Macromol. 2019, 137, 992–1001.
- Dai, J.; Tian, Q.; Sun, Q.; Wei, W.; Zhuang, J.; Liu, M.; Cao, Z.; Xie, W.; Fan, M. TiO2-alginate composite aerogels as novel oil/water separation and wastewater remediation filters. Compos. Part B Eng. 2019, 160, 480–487.
- Abdel Rehim, M.H.; El-Samahy, M.A.; Badawy, A.A.; Mohram, M.E. Photocatalytic activity and antimicrobial properties of paper sheets modified with TiO2/Sodium alginate nanocomposites. Carbohydr. Polym. 2016, 148, 194–199.
- Papageorgiou, S.K.; Katsaros, F.K.; Favvas, E.P.; Romanos, G.E.; Athanasekou, C.P.; Beltsios, K.G.; Tzialla, O.I.; Falaras, P. Alginate fibers as photocatalyst immobilizing agents applied in hybrid photocatalytic/ultrafiltration water treatment processes. Water Res. 2012, 46, 1858–1872.
- Khodadadi, B. Facile sol–gel synthesis of Nd, Ce-codoped TiO2 nanoparticle using starch as a green modifier: Structural, optical and photocatalytic behaviors. J. Sol-Gel Sci. Technol. 2016, 80, 793–801.
- Lin, D.; Huang, Y.; Liu, Y.; Luo, T.; Xing, B.; Yang, Y.; Yang, Z.; Wu, Z.; Chen, H.; Zhang, Q.; et al. Physico-mechanical and structural characteristics of starch/polyvinyl alcohol/nano-titania photocatalytic antimicrobial composite films. Lwt 2018, 96, 704–712.
- Muniandy, S.S.; Mohd Kaus, N.H.; Jiang, Z.T.; Altarawneh, M.; Lee, H.L. Green synthesis of mesoporous anatase TiO2 nanoparticles and their photocatalytic activities. RSC Adv. 2017, 7, 48083–48094.
- Warkhade, S.K.; Gaikwad, G.S.; Zodape, S.P.; Pratap, U.; Maldhure, A.V.; Wankhade, A.V.L. Temperature synthesis of pure anatase carbon doped titanium dioxide: A. efficient visible light active photocatalyst Low temperature synthesis of pure anatase carbon doped titanium dioxide: An efficient visible light active photocatalyst. Mater. Sci. Semicond. Process. 2017, 63, 18–24.
- Toor, A.P.; Yadav, N.; Wanchoo, R.K. Enhancement in photocatalytic activity of nano-TiO2 photocatalyst by carbon doping. Mater. Sci. Forum 2013, 757, 271–284.
- Bobirică, C.; Bobirică, L.; Râpă, M.; Matei, E.; Predescu, A.M.; Orbeci, C. Photocatalytic degradation of ampicillin using PLA/TiO2 hybrid nanofibers coated on different types of fiberglass. Water 2020, 12, 176.
- Eleftheriadou, N.M.; Ofrydopoulou, A.; Papageorgiou, M.; Lambropoulou, D. Development of novel polymer supported nanocomposite GO/TiO2 films, based on poly(L-lactic acid) for photocatalytic applications. Appl. Sci. 2020, 10, 2368.
- Evgenidou, E.; Ofrydopoulou, A.; Malesic-Eleftheriadou, N.; Nannou, C.; Ainali, N.M.; Christodoulou, E.; Bikiaris, D.N.; Kyzas, G.Z.; Lambropoulou, D.A. New insights into transformation pathways of a mixture of cytostatic drugs using Polyester-TiO2 films: Identification of intermediates and toxicity assessment. Sci. Total Environ. 2020, 741.
- Xie, J.; Hung, Y.C. UV-A activated TiO2 embedded biodegradable polymer film for antimicrobial food packaging application. Lwt 2018, 96, 307–314.
- Zhu, Y.; Piscitelli, F.; Buonocore, G.G.; Lavorgna, M.; Amendola, E.; Ambrosio, L. Effect of surface fluorination of TiO2 particles on photocatalitytic activity of a hybrid multilayer coating obtained by sol-gel method. ACS Appl. Mater. Interfaces 2012, 4, 150–157.
- Liu, M.; Cheng, Z.; Yan, J.; Qiang, L.; Ru, X.; Liu, F.; Ding, D.; Li, J. Preparation and characterization of TiO2 nanofibers via using polylactic acid as template. J. Appl. Polym. Sci. 2013, 128, 1095–1100.
- Sökmen, M.; Tatlidil, I.; Breen, C.; Clegg, F.; Buruk, C.K.; Sivlim, T.; Akkan, Ş. A new nano-TiO2 immobilized biodegradable polymer with self-cleaning properties. J. Hazard. Mater. 2011, 187, 199–205.
- Park, S.Y.; Lee, H.U.; Ahn, K.; Kim, J.P.; Jin, J.S.; Lee, J.; Jeong, S.Y.; Cho, C.R. Enhanced photocatalytic activity of TiO2-incorporated nanofiber membrane by oxygen plasma treatment. Thin Solid Films 2011, 519, 6899–6902.
- Tu, H.; Li, D.; Yi, Y.; Liu, R.; Wu, Y.; Dong, X.; Shi, X.; Deng, H. Incorporation of rectorite into porous polycaprolactone/TiO2 nanofibrous mats for enhancing photocatalysis properties towards organic dye pollution. Compos. Commun. 2019, 15, 58–63.
- Karagoz, S.; Kiremitler, N.B.; Sakir, M.; Salem, S.; Onses, M.S.; Sahmetlioglu, E.; Ceylan, A.; Yilmaz, E. Synthesis of Ag and TiO2 modified polycaprolactone electrospun nanofibers (PCL/TiO2-Ag NFs) as a multifunctional material for SERS, photocatalysis and antibacterial applications. Ecotoxicol. Environ. Saf. 2020, 188.
- Sivlim, T.; Akkan, Ş.; AltIn, I.; Koç, M.; Sökmen, M. TiO2 immobilized biodegradable polymer for photocatalytic removal of chlorophenol. Water. Air. Soil Pollut. 2012, 223, 3955–3964.
- Marković, D.; Milovanović, S.; Radovanović, Ž.; Zizovic, I.; Šaponjić, Z.; Radetić, M. Floating Photocatalyst Based on Poly(ε-caprolactone) Foam and TiO2 Nanoparticles for Removal of Textile Dyes. Fibers Polym. 2018, 19, 1219–1227.
- Kreetachat, T.; Kruenate, J.; Suwannahong, K. Preparation of TiO2/Bio-composite film by sol-gel method in VOCs photocatalytic degradation process. Appl. Mech. Mater. 2013, 390, 552–556.
- Pelaseyed, S.S.; Hosseini, H.R.M.; Nokhbedehghan, Z.; Samadikuchaksaraei, A. PLGA/TiO2 nanocomposite scaffolds for biomedical applications: Fabrication, photocatalytic, and antibacterial properties. BioImpacts 2020, 11, 45–52.
- Korina, E.; Stoilova, O.; Manolova, N.; Rashkov, I. Multifunctional hybrid materials from poly(3-hydroxybutyrate), TiO2 nanoparticles, and chitosan oligomers by combining electrospinning/electrospraying and impregnation. Macromol. Biosci. 2013, 13, 707–716.
