The use of urface-engineered coatings for the fire protection of cotton fabrics is continously growing. In this context, two main approaches have been extensively investigated, namely sol-gel derived coatings and layer-by-layer assemblies. These approaches are both capable of providing treated fabrics with outstanding flame-retardant features, when exposed to a flame or an irradiative heat flux. This review work aims at discussing the recent progresse with respect to both strategies, highlighting current limitations, open challenges, and possible further developments.
- cotton
- surface engineering
- sol-gel
- layer-by-layer
- coatings
- flame retardance
- flame spread tests
- forced-combustion tests
- durability
In general, the high flammability of fibers and fabrics significantly limits their exploitation in those sectors where fire-proofing is mandatory. In fact, if not properly modified, textile materials burn very easily when exposed to a flame or an irradiative heat flux; subsequent smoke generation can further cause severe issues because of toxicity.
In order to overcome this issue, in the early 1950s flame retardants (FRs), i.e., materials that are able to inhibit or at least slow down the propagation of a flame, were proposed. Nowadays, some of these are commercially available products with a high effectiveness according to the specific chemical and/or physical mechanisms that take place when FRs activate [
,
]. Generally speaking, flaming combustion is a “simple” oxidative reaction that occurs in the gas phase in the presence of oxygen (or air) supplied by the atmosphere surrounding the burning material. The degrading material generates combustible gaseous species mixed together with atmospheric oxygen, hence starting a combustion process which is self-sustained by the exothermicity of the flame (
).
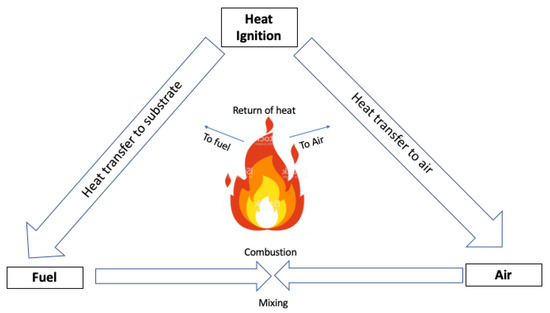
A self-sustaining combustion cycle (i.e., Emman’s fire triangle) for flammable materials.The chemical composition and structure of the FRs play a key role in determining the way through which the FRs can be active in the condensed or gas phase; in addition, overall flame retardance is strictly related to the structure and morphology of the flame-retarded system, as well as to its thermal and fire behavior [
,
,
].
For an effective flame-retardant action, all FRs should contain some specific elements in their chemical structure (namely halogens, boron, nitrogen, phosphorus, and silicon, alone or in combination), so that synergistic effects can be possibly exploited. For bulky polymers, FRs can be incorporated utilizing different techniques: in particular, melt compounding is very often exploited; in some other cases FRs can be covalently linked to the polymer by means of grafting or copolymerization reactions [
5].
Conversely, textile materials show a very irregular surface; aside from some synthetic materials, which can be flame-retarded by incorporating specific additives into the polymer bulk before spinning, flame retardance is very often provided by using surface-engineered methods. In particular, the fibers and fabrics can be treated with solutions or suspensions (possibly waterborne, in order to avoid the use of toxic or environmentally unsound organic solvents) containing flame retardants. This way, it is possible to utilize the already available industrial impregnation/exhaustion lines. Then, specifically referring to fabric substrates, it is also possible to coat them with one or more flame-retardant layers (that can be continuous or not) on the side that will be exposed to the flame or the irradiative heat source alone, or on both sides. The optimization of the deposited layers allows for the best performance in fire [
6,
7].].
It is noteworthy that all these surface methods take advantage of the presence of flame retardants on the surface of the material for protection. In fact, the surface is the first to interact with the flame or the irradiative heat flux and therefore it is this, rather than the bulk, that has to be well protected.
Besides, the design of any flame retardant for textile materials has to fulfill the following conditions:
- Regarding the mechanism taking place in the condensed phase, the pyrolysis of the flame-retarded textile should promote a charring effect, limiting the production of combustible volatiles. This is unfavorable for continued combustion; in addition, it may be possible to exploit an intumescent effect provided by the flame-retarding treatment on the basis of the chemical structure and composition of the FR additive;
-
The heat that is re-directed towards the textile should be restricted as much as possible: this way, it is possible to limit pyrolysis reactions that, in turn, may favor the formation of combustible volatiles;
-
The FR treatment should not impact on the durability, softness of touch, dyeability, aesthetics, and even the external appearance of the treated fabrics.
- The exothermicity of the reactions occurring during the interaction of the flame-retarded textile with the flame or heat source should be lower than that needed for maintaining the combustion process;
- Regarding the mechanism taking place in the condensed phase, the pyrolysis of the flame-retarded textile should promote a charring effect, limiting the production of combustible volatiles. This is unfavorable for continued combustion; in addition, it may be possible to exploit an intumescent effect provided by the flame-retarding treatment on the basis of the chemical structure and composition of the FR additive;
- The heat that is re-directed towards the textile should be restricted as much as possible: this way, it is possible to limit pyrolysis reactions that, in turn, may favor the formation of combustible volatiles;
- The FR treatment should not impact on the durability, softness of touch, dyeability, aesthetics, and even the external appearance of the treated fabrics.
-
The exothermicity of the reactions occurring during the interaction of the flame-retarded textile with the flame or heat source should be lower than that needed for maintaining the combustion process;
From a technical point of view, some halogenated flame retardants (namely polychlorinated biphenyls and decabromodiphenyl or pentabromodiphenyl ethers) were found to be very efficient but at the same time toxic, and were therefore banned [
8,
9,
10,
11], hence pushing academic and industrial research towards the search for low-environmental-impact alternatives. In this context, phosphorus-based and/or phosphorus/nitrogen-containing additives were developed for the replacement of halogenated counterparts [
12].].Specifically referring to the flame retardance of cotton, a good commercial halogen-free solution was identified thanks to the setup of flame retardants for coatings and back-coated fabrics or to the design and production of Proban
®and Pyrovatex
®, which are based on hydroxymethylphosphonium salts and N-methylol phosphonopropionamide derivatives, respectively. These two latter derivatives show a high FR effectiveness, but also significant limitations. In particular, Pyrovatex
®allows for linking almost 50% of the flame retardant to the fabric surface; therefore, the rest is lost during the first washing cycle. Regarding Proban
®, its application to the cellulosic textile requires a specific industrial plant process; in addition, formaldehyde may be released during the use of the flame-retarded material [
13].
Though it is not easy to design and develop efficient and low-environmental-impact flame retardants, two surface engineering approaches have shown high potentialities, especially over the last 10 years: the first one is the sol-gel technique, a well-established method that appeared in the 1950s for synthesizing ceramic materials; the second is the layer-by-layer (LbL) method, which appeared more recently than sol-gel as far as its application to fabrics is considered. Both approaches can be considered as effective ways to provide fabrics with multifunctional features, i.e., not only with flame-retardant properties, but also with hydrophobicity, antibacterial activity, and electrical conductivity, among other characteristics, depending on the chemical structure of the sol-gel precursors or of the deposited layer-by-layer (LbL) architectures [
6,
7].
From a general point of view, the sol-gel technique is very suitable for application to textiles, considering their irregular surface topography; besides, its industrial application can be carried out using already existing finishing lines. Furthermore, the high number of hydroxyl functionalities on the cellulosic substrate can be successfully utilized for covalently linking the sol-gel coating to the fabrics, hence improving their durability (i.e., resistance to washing treatments which textiles are very often subjected to).
The layer-by-layer (LbL) technique was discovered in the 1960s by Iler [
14], who first proved the suitability of the process for assembling nanostructured layers through a molecularly-controlled method. Surprisingly, the LbL technique was abandoned until the early 1990s, when a useful methodology for producing nanoarchitectures consisting of layers of anionic and cationic polyelectrolytes on a substrate was proposed [
15]. As a result, assembly deposited on fabrics does not affect their bulk mechanical behavior, providing, at the same time, useful features that are strictly dependent on the type and number of deposited layers [
16,
17,
18].].
