Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Fabian Hemm.
The rodent model of experimental autoimmune-orchitis (EAO) was established to analyze the underlying mechanisms of male infertility and causes of reduced testosterone concentration.
- experimental autoimmune-orchitis
- osteoporosis
- mouse model
- biomechanical properties
- rodent model
- Complete Freund’s Adjuvant
- Bordetella pertussis toxin
1. Introduction
Testicular inflammation is mainly caused by bacterial infections, either sexually transmitted or from the urinary tract, and often develops a chronic asymptomatic disease progression [1][2][3][4]. Therefore, orchitis can persist over prolonged periods until adequate therapy is started and may result in irreversible long-term damage regarding the reproductive system [2][3]. In this regard, inflammatory infiltrates in the testicular interstitium, damaged seminiferous tubules and disorder of spermatogenesis have been illustrated [2]. In most cases inflammation affects epididymides as well, and leads to a combined epididymo-orchitis [1][2]. Consequently, orchitis, or rather epididymo-orchitis, represents a frequent cause of fertility disorders in young men [3][5]. However, the majority of fertility disorders are diagnosed a long time after the initial inflammatory process. Thus, in vitro and in vivo models are crucial to investigate the underlying processes of epididymo- orchitis and its complications. In this context the rodent model of experimental autoimmune-orchitis (EAO) represents a well-established animal model to evaluate the processes of testicular inflammation, consequences regarding the reproductive functionality and possible therapy options [6][7][8][9]. Furthermore, EAO was found to significantly reduce the testosterone concentration in rats in a similar way to human orchitis [6]. Androgens are known to represent a major impact factor regarding bone metabolism in men [10][11][12]. Accordingly, huma study fon studies illustrated the development of osteoporosis even in young men suffering from hypogonadism [13][14].
2. Histomorphometric Bone Architecture
Microscopic overviews of hematoxylin-eosin-stained vertebral bodies (Figure 1) were automatically evaluated regarding bone area (B.Ar), trabecular thicknesed on ts (Tb.Th) and trabecular separation (Tb.Sp), while the trabecular perimeter (Tb.Pm) was measured manually. Histomorphometrical measurement illustrated a significantly decreased bone area due to EAO (0.361 ± 0.020 mm2) and nearly significant reduction in the adjuvant group (Adj; 0.453 ± 0.043 mm2) compared to the control group (Cont; 0.594 ± 0.048 mm2, p < 0.01 vs. EAO, p = 0.080 vs. Adj, Figure 2). However, trabecular perimeter (EAO 17.082 ± 1.190 mm, Adj 17.358 ± 0.658 mm, Cone status in a t 21.550 ± 2.274 mm), trabecular thickness (EAO 17.050 ± 1.942 µm, Adj 17.509 ± 0.904 µm, Cont 18.024 ± 0.971 µm) and trabecular separation (EAO 106.298 ± 12.552 µm, Adj 108.196 ± 3.266 µm, Cont 87.265 ± 6.338 µm) remained without significant alterations.
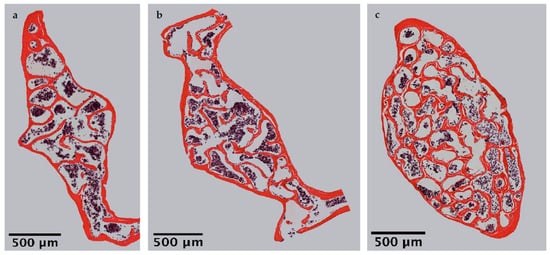
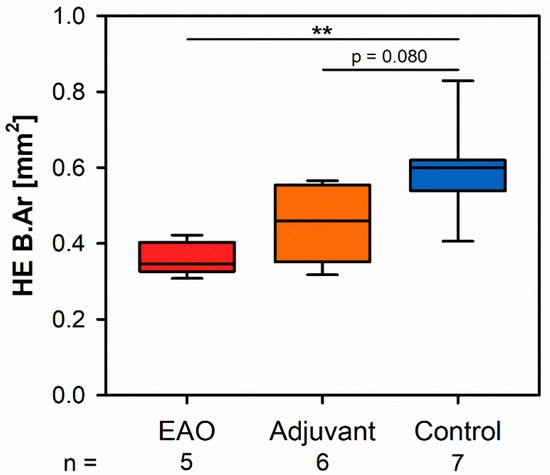

Figure 1. Microscopic overviews of vertebral bodies L3 stained with hematoxylin-eosin. Images taken at 40× magnification were merged. Mice were immunized with (a) testicular homogenate in adjuvant (EAO), (b) adjuvant alone or (c) remained untreated.

Figure 2. Histomorphometrical results regarding the bone area (B.Ar) in vertebral bodies L3 stained with hematoxylin-eosin (HE). Mice were immunized with testicular homogenate in adjuvant (EAO), adjuvant alone (adjuvant) or remained untreated (control). ** p < 0.01.
3. Microarchitecture of Cortical Bone
The microuse model oarchitecture of the femoral cortex was analyzed at mid diaphysis and distal diaphysis by micro computed tomography (µCT). Two-dimensional example images from µCT scans (Figure 3) demonstrated a loss of cortical as well as trabecular bone mass following (a) EAO ilor (b) treatment with adjuvant alone, compared to (c) untreated mice.
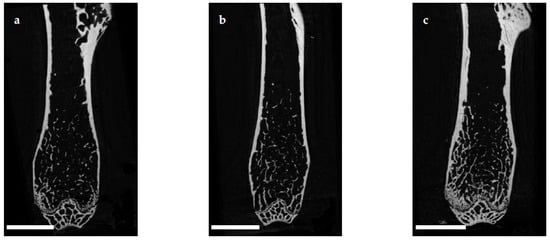

Figure 3. Two-dimensional images from µCT scans of distal femora (scale bar 2 mm). Mice were immunized with (a) testicular homogenate in adjuvant (EAO), (b) adjuvant alone or (c) remained untreated.
At the mid diaphyseal region, µCT identified a significantly redustced cortical thickness (Ct.Th, Figure 4a) in the EAO (0.147 ± 0.005 mm) and adjuvant group (0.155 ± 0.005 mm) in contratest to the control group (0.210 ± 0.007 mm, p < 0.001 each) with no difference sibetween EAO and adjuvant. The cortical surface (Ct.S, not graphically displayed) remained unaltered in EAO (10.538 ± 0.163 mm2) compared to control (10.270 ± 0.235 mm2, p = 1.000), but slightly decreased in the adjuvant group (9.530 ± 0.238 mm2, p < 0.05 vs. EAO, p = 0.087 vs. Cont). In contrast, the cortical surfiace/volume ratio (Ct.S/Ct.V, Figure 4b) was significantly aincreased in EAO (18.588 ± 0.608 mm−1) and adjuvant (17.594 ± 0.549 mm−1) compared to control (13.670 ± 0.326 mm−1, p < 0.001 each). Therefore, therations regardi cortical surface/volume ratio indirectly indicated a loss of cortical bone volume in EAO and adjuvant without significant differences between them. The bone mineral density (BMD, Figure 4c) was reduced in EAO (1.164 ± 0.010 g/cm3, p < 0.001 vs. Cont 1.280 ± 0.006 g/cm3) while adjuvant (1.270 ± 0.045 g/cm3) showed no difference to control. Similarly, the tissue mineral density (TMD, Figure 4d) was decreased in the EAO group (1.480 ± 0.004 g/cm3) compared to control (1.537 ± 0.005 g/cm3, p < 0.001) and almost significantly decreased compared to the adjuvant group (1.531 ± 0.015 g/cm3, p = 0.053 vs. EAO) with no difference between adjuvant and control (p = 0.974).
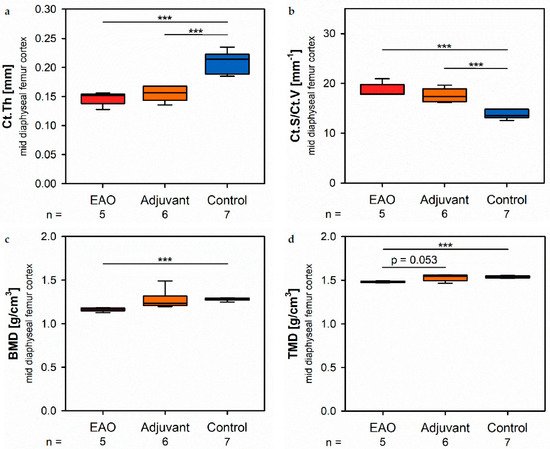

Figure 4. Microarchitecture of mid diaphyseal femur cortex in µCT. Measurement of (a) cortical thickness (Ct.Th), (b) cortical surface/volume ratio (Ct.S/Ct.V), (c) bone mineral density (BMD) and (d) tissue mineral density (TMD). Mice were immunized with testicular homogenate in adjuvant (EAO), adjuvant alone (adjuvant) or remained untreated (control). *** p < 0.001.
At the distal diaphysis, µCT showed a significantly thinner cortical thickness (Figure 5a) in the EAO (0.113 ± 0.004 mmet) and adjuvant group (0.125 ± 0.005 mm) compared to the control group (0.165 ± 0.002 mm, p < 0.001 each) similar to the mid diaphysis. The cortical surface (not graphically displayed) remained without differences in all groups (EAO 13.012 ± 0.274 mm2, Adj 11.556 ± 0.326 mm2, Cont 12.686 ± 0.438 mm2). Again, the cortical surface/volume ratio (Figure 5b) was significantly increased in the EAO (24.713 ± 0.982 mm−1) and adjuvant group (22.396 ± 0.915 mm−1) in contrast to control (17.706 ± 0.152 mm−1, p < 0.01 each) and indicated a lossm of cortical bone volume indirectly. The bone mineral density (Figure 5c) was reduced in EAO (0.944 ± 0.015 g/cm3) compared to control (1.055 ± 0.005 g/cm3, p < 0.001). Additionally, bone minerassl density at distal diaphysis was also decreased in the adjuvant group (0.993 ± 0.020 g/cm3, p < 0.05) unlike at the mid diaphysis. In contrast, the tissue mineral density (Figure 5d) was only reduced in the EAO group (1.273 ± 0.012 g/cm3) compared to control (1.319 ± 0.005 g/cm3, p < 0.05) while adjuvant remained unaltered (1.309 ± 0.017 g/cm3, p = 1.000).
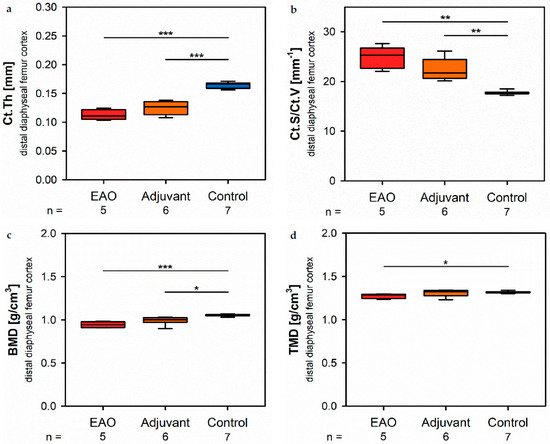

Figure 5. Microarchitecture of distal diaphyseal femur cortex in µCT. Measurement of (a) cortical thickness (Ct.Th), (b) cortical surface/volume ratio (Ct.S/Ct.V), (c) bone mineral density (BMD) and (d) tissue mineral density (TMD). Mice were immunized with testicular homogenate in adjuvant (EAO), adjuvant alone (adjuvant) or remained untreated (control). * p < 0.05; ** p < 0.01; *** p < 0.001.
4. Microarchitecture of Trabecular Bone
The microarchitecture of trabecular bone microwas studied in femora and lumbar vertebral bodies (L1) by µCT. Two-dimensional example images from µCT scans of vertebral bodies (Figure 6) illustrate a loss of trchiteabecular bone mass following (a) EAO or (b) treatment with adjuvant alone compared to (c) untreated mice, similar to µCT scans of the distal femora (Figure 3).
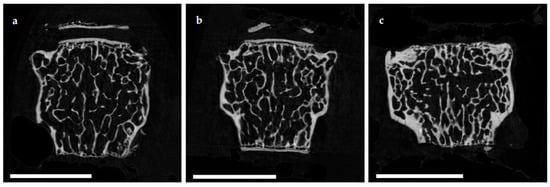

Figure 6. Two-dimensional images from µCT scans of vertebral bodies L1 (scale bar 2 mm). Mice were immunized with (a) testicular homogenate in adjuvant (EAO), (b) adjuvant alone or (c) remained untreated.
In the trabecular region of ture he femora, µCT indicated a loss of trabecular number (Tb.N, Figure 7a) and btrabecular thickness (Tb.Th, Figure 7b) in EAO (Tb.N: 1.171 ± 0.099 mm−1; Tb.Th: 0.038 ± 0.003 mm) and in the adjuvant group (Tb.N: 1.344 ± 0.133 mm−1; Tb.Th: 0.038 ± 0.002 mm) compared to control (Tb.N: 2.771 ± 0.197 mm−1, p < 0.001 each; mTb.Th: 0.048 ± 0.002 mm, p < 0.05 each). Trabecular separation (Tb.Sp, Figure 7c) was larger in EAO (0.276 ± 0.006 mm) alnd adjuvant (0.256 ± 0.005 mm) than in control (0.199 ± 0.006 mm, p < 0.001 each). Consequently, the bone volume fraction (BV/TV, Figure 7d), as well as the bone surface density (BS/TV, Figure 7e) were significantly decreased in EAO (BV/TV: 4.452 ± 0.447%; BS/TV: 4.427 ± 0.335 mm−1) and adjuvant groups (BV/TV: 5.065 ± 0.566%; BS/TV: 5.171 ± 0.440 mm−1) in comparison to control (BV/TV: 13.340 ± 1.283%, p < 0.01 each; BS/TV: 10.340 ± 0.698 mm−1, p < 0.001 each). Thereby, the specific bone surface (BS/BV, Figure 7f) increased in the EAO (101.214 ± 6.639 mm−1, p < 0.05 vs. Cont 79.430 ± 3.681 mm−1) and adjuvant groups (103.878 ± 4.293 mm−1, p < 0.01). This finding illustrates a pronounced loss of trabecular bone volume over trabecular bone surface. Furthermore, the structure model index (SMI; Figure 7g) and trabecular pattern factor (Tb.Pf, not graphically displayed) were significantly elevated in EAO (SMI: 2.252 ± 0.087; Tb.Pf: 37.822 ± 2.271 mm−1) and adjuvant (SMI: 2.231 ± 0.100; Tb.Pf: 38.736 ± 2.703 mm−1) compared to control (SMI: 1.699 ± 0.102, p < 0.01 each; Tb.Pf: 22.782 ± 2.360 mm−1, p < 0.01 each), both indicating a degradation of trabecular integrity. The bone mineral density (BMD, Figure 7h) was reduced in both immunized groups (EAO 0.078 ± 0.006 g/cm3, Adj 0.091 ± 0.008 g/cm3) in contrast to control (0.205 ± 0.016 g/cm3, p < 0.001 each) similar ton the femoral cortex. Equally, the tissue mineral density (TMD, Figure 7i) was decreasued in both immunized groups (EAO 0.922 ± 0.018 g/cm3, Adj 0.931 ± 0.012 g/cm3) compared to control (1.002 ± 0.012 g/cm3, p < 0.01 each).
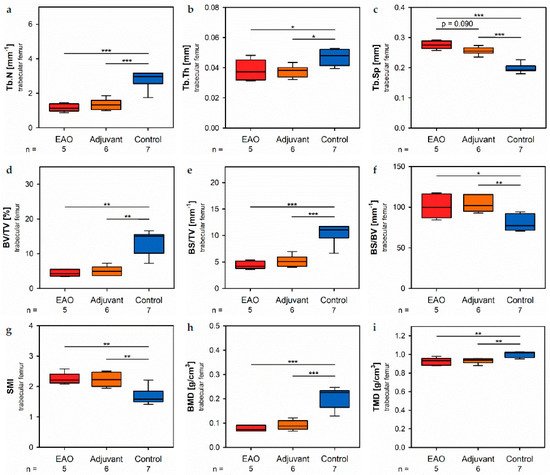

Figure 7. Microarchitecture of trabecular femur by µCT. Measurement of (a) trabecular number (Tb.N), (b) trabecular thickness (Tb.Th), (c) trabecular separation (Tb.Sp), (d) bone volume fraction (BV/TV), (e) bone surface density (BS/TV), (f) specific bone surface (BS/BV), (g) structure model index (SMI), (h) bone mineral density (BMD) and (i) tissue mineral density (TMD). Mice were immunized with testicular homogenate in adjuvant (EAO), adjuvant alone (adjuvant) or remained untreated (control). * p < 0.05; ** p < 0.01; *** p < 0.001.
Investigationg in de of the trabecular region in vertebral bodies L1 by µCT (Figure 8) demonstrated generally similar alterations like the trabecularea region in femora (Figure 7). However, several significant d biomechaifferences between EAO and the adjuvant group were detected in the trabecular region of vertebral bodies L1, while both groups were mostly comparable in the trabecular region of the femora.
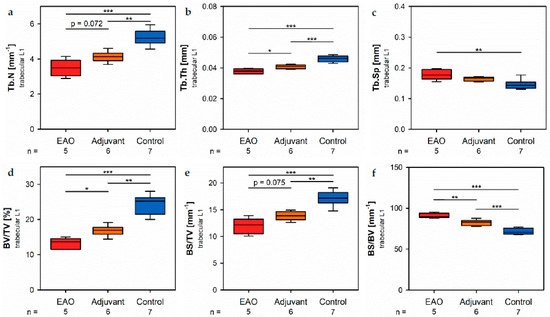
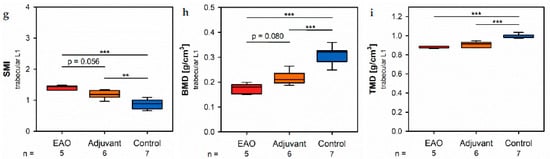


Figure 8. Microarchitecture of trabecular vertebral body L1 by µCT. Measurement of (a) trabecular number (Tb.N), (b) trabecular thickness (Tb.Th), (c) trabecular separation (Tb.Sp), (d) bone volume fraction (BV/TV), (e) bone surface density (BS/TV), (f) specific bone surface (BS/BV), (g) structure model index (SMI), (h) bone mineral density (BMD) and (i) tissue mineral density (TMD). Mice were immunized with testicular homogenate in adjuvant (EAO), adjuvant alone (adjuvant) or remained untreated (control). * p < 0.05; ** p < 0.01; *** p < 0.001.
In the vertebral bodicaes, L1 trabecular number (Figure 8a) and trabecular sthickness (Figure 8b) were significantly decreabsed in the EAO (Tb.N: 3.488 ± 0.215 mm−1; Tb.Th: 0.038 ± 0.001 mm) and adjuvant groups (Tb.N: 4.131 ± 0.123 mm−1; Tb.Th: 0.041 ± 0.001 mm) compared to control (Tb.N: 5.243 ± 0.176 mm−1, p < 0.001 vs. EAO, p < 0.01 vs. Adj; Tb.Th: 0.046 ± 0.001 mm, p < 0.001 each). Furthermore, trabecular number was nearly (p = 0.072) and trabecular thickness significantly (p < 0.05) lower in EAO than in the adjuvant group. The trabecular separation (Figure 8c) was larger following EAO (0.179 ± 0.008 mm) than in the control group (0.147 ± 0.006 mm, p < 0.01), but unaltered in the adjuvant group (0.164 ± 0.003 mm, p = 0.113). These findings resulted in a significant reduction of the bone volume fraction (Figure 8d) as well as the bone surface density [13] (Figure 8e) in EAO (BV/TV: 13.154 ± 0.711%; BS/TV: 11.951 ± 0.659 mm−1) and in the adjuvant group (BV/TV: 16.856 ± 0.628%; BS/TV: 13.868 ± 0.342 mm−1) in comparison to control (BV/TV: 24.056 ± 1.132%, p < 0.001 vs. EAO, p < 0.01 vs. Adj; BS/TV: 17.149 ± 0.535 mm−1, p < 0.001 vs. EAO, p < 0.01 vs. Adj). Bone volutme fraction was significantly lower (p < 0.05), andue to bone surface density was almost lower (p = 0.075) in the EAO than in the adjuvant group. The specific bone surface (Figure 8f) was increased in the adjuvant group (82.504 ± 1.489 mm−1, p < 0.001 vs. Cont 71.647 ± 1.474 mm−1) and even more significantly araised after EAO (90.882 ± 1.376 mm−1, p < 0.001 vs. Cont, p < 0.01 vs. Adj), again indicating a predominant loss of teratirabecular bone volume over trabecular bone surface. Both the structure model index (Figure 8g) and trabecular pattern factor (ns fot graphically displayed) illustrated a loss of trabecular integrity in EAO (SMI: 1.400 ± 0.033; Tb.Pf: 21.194 ± 0.562 mm−1) and adjuvant groups (SMI: 1.188 ± 0.054; Tb.Pf: 16.390 ± 1.011 mm−1) compared to control (SMI: 0.876 ± 0.061, p < 0.001 vs. EAO, p < 0.01 vs. Adj; Tb.Pf: 10.544 ± 0.927 mm−1, p < 0.001 vs. EAO, p < 0.01 vs. Adj). Again, EAO was more severely affected in this regard than the adjuvant group (SMI: p = 0.056; Tb.Pf: p < 0.01). The bone mineral density (Figure 8h) was decreased ing both immunized groups (EAO 0.173 ± 0.009 g/cm3, Adj 0.216 ± 0.011 g/cm3) in contrast to control (0.308 ± 0.014 g/cm3, p < 0.001 each). Likewise, the tissue mineral density (Figure 8i) was reduced in EAO (0.883 ± 0.004 g/cm3) and adjuvant groups (0.908 ± 0.012 g/cm3) compared to control (0.995 ± 0.008 g/cm3, p < 0.001 each).
5. Osteoblasts
Osteoblast activity was determined by the expression of alkaline phosphatase (ALP), a major enzation yme of osteoblast hydroxyapatite synthesis. Enzyme histochemical staining of ALP in vertebral bodies resulted in a distinctive violet labeling located along the trabecular surface (Figure 9a). Histomorphometrical calculation illustrated a significantly decreased relative ALP perimeter (ALP.Pm/Tb.Pm, Figure 9b) in the EAO (12.720 ± 1.841%) and adjuvant groups (21.298 ± 3.237%) compared to control (43.185 ± 4.818%, p < 0.001 vs. EAO, p < 0.01 vs. Adj). This finding was supported by a reduced mRNA expression of ALP (Figure 9c) in the complete Freund’s adjuvant (immunized groups (EAO −9.034 ± 0.102 [−∆CP], Adj −9.017 ± 0.106 [−∆CP]) in contrast to control (−8.461 ± 0.110 [−∆CP], p < 0.01 each) determined by real-time reverse transcription polymerase chain reaction (real-time RT-PCR). Beside bone mineralization, osteoblasts are responsible for synthesis and secretion of organic bone matrix. Therefore, we examined the mRNA expression of collagen 1α1 (Col1α1, Figure 9d) as another parameter of osteoblast activity. Regarding the mRNA expression of collagen 1α1, we detected no significant differences between the investigated groups (EAO −2.092 ± 0.256 [−∆CFP], A) alone, tdj −2.490 ± 0.082 [−∆CP], Cont −2.411 ± 0.143 [−∆CP]). Additionally, we conducted Von Kossa/Van Gieson staining of histological specimens, which resulted in a red labeling of osteoid (Figure 9f). However, the histomouse orphometrical calculation of relative osteoid area (O.Ar/B.Ar, Figure 9e) influenced neither EAO nor adjuvant compared tod control (EAO 21.674 ± 1.662%, Adj 17.720 ± 4.299%, Cont 15.695 ± 3.673%).
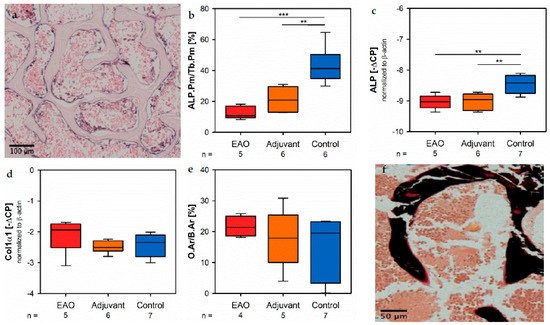

Figure 9. Osteoblasts. (a) Enzyme histochemical staining of alkaline phosphatase (ALP, violet) in vertebral body L3 from an untreated mouse. (b) Histomorphometrical calculation of relative ALP perimeter (ALP.Pm/Tb.Pm) in enzyme histochemical-stained vertebral bodies L3. mRNA expression of (c) ALP and (d) collagen 1α1 (Col1α1) in real-time RT-PCR of vertebral bodies Th10. (e) Histomorphometrical calculation of relative osteoid area (O.Ar/B.Ar) in vertebral bodies L2 by Von Kossa/Van Gieson staining. (f) Von Kossa/Van Gieson staining in vertebral body L2 from an untreated mouse (red: osteoid; black: mineralized bone; brown: bone marrow). Mice were immunized with testicular homogenate in adjuvant (EAO), adjuvant alone (adjuvant) or remained untreated (control). ** p < 0.01; *** p < 0.001.
6. Osteoclasts
Osteocl of asts were identified by enzyme histochemical staining of tartrate resistant acid phosphatase (TRAP). Multinuclear, red stained cells in contact to the trabecular surface were counted as osteoclasts (Figure 10a). The calculation of osteoclast number in relation to trabecular perimeter (TRAP.N/Tb.Pm, Figure 10b) illustrated a significant reduction of osteoclasts in EAO (1.924 ± 0.488 n/mm) and adjuvant groups (1.933 ± 0.407 n/mm) compared to control (5.447 ± 0.527 n/mm, p < 0.001 each). These findings were supplemented by the rrently appesults of real-time RT-PCR regarding cathepsin K (CtsK), another typical degrading enzyme of osteoclasts. The mRNA expression of cathepsin K (Figure 10c) was significantly decrears to sed after EAO (−4.522 ± 0.292 [−∆CP]) in comparison to control (−3.754 ± 0.159 [−∆CP], p < 0.05) while it was not significantly decreased in the adjuvant group (−4.097 ± 0.129 [−∆CP], p = 0.620). As a major regulatory factor of osteoclast differentiation, we determined the mRNA expression of the receptor activator of nuclear factor-kappa B ligand (RANKL, Figure 10d) but no significant alterations were inapprodetected (EAO −11.292 ± 0.088 [−∆CP], Adj −11.192 ± 0.085 [−∆CP], Cont −10.816 ± 0.237 [−∆CP]). Additionally, we evaluated the mRNA expression of osteoprotegerin (OPG, Figure 10e), an inhibitor of RANKL and, thereby, regulating the differentiation of osteoclasts. Expressiate to on of osteoprotegerin was significantly reduced in the adjuvant group (−10.530 ± 0.186 [−∆CP], p < 0.01 vs. Cont −9.493 ± 0.194 [−∆CP]) and almost significantly decreased after EAO (−10.262 ± 0.249 [−∆CP], p = 0.062 vs. Cont). Therefore, the reduction of osteoclast activity can neither been explained by RANKL nor by osteoprotegerin.
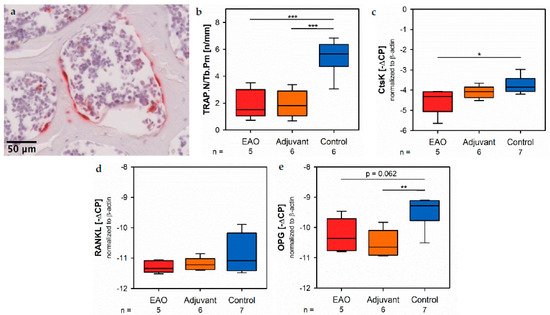

Figure 10. Osteoclasts. (a) Enzyme histochemical staining of tartrate resistant acid phosphatase (TRAP, red) in vertebral body L3 from an untreated mouse. (b) Histomorphometrical calculation of number of TRAP positive cells per trabecular perimeter (TRAP.N/Tb.Pm) in enzyme histochemical-stained vertebral bodies L3. mRNA expression of (c) cathepsin K (CtsK), (d) receptor activator of nuclear factor-kappa B ligand (RANKL) and (e) osteoprotegerin (OPG) in real-time RT-PCR of vertebral bodies Th10. Mice were immunized with testicular homogenate in adjuvant (EAO), adjuvant alone (adjuvant) or remained untreated (control). * p < 0.05; ** p < 0.01; *** p < 0.001.
7. Osteocytes and Cell-Contacts
Since sclerostin represents a typical marker of osteocytes, we analyzed the mRNA expressine ton of the sclerostin gene (SOST, Figure 11a) by real-time RT-PCR. The mRNA effects oxpression of SOST was significantly decreased in both immunized groups (EAO −12.782 ± 0.277 [−∆CP], Adj −12.522 ± 0.281 [−∆CP]) compared to control (−11.359 ± 0.295 [−∆CP], p < 0.05 each). Gap junctions link osteocytes with each other and are mandatory regarding structural integrity and cellular survival. Connexin 43 represents a characteristic element of gap junctiorcns. The mRNA expression of connexin 43 (Cx43, Figure 11b) was significantly lower after EAO (−8.530 ± 0.164 [−∆CP]) than iti was in the control group (−7.759 ± 0.122 [−∆CP], p < 0.01) while the adjuvant group showed rean intermediate mRNA level of connexin 43 (−8.100 ± 0.097 [−∆CP], p = 0.199 vs. Cont).
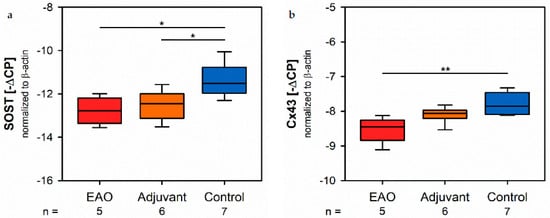

Figure 11. Osteocytes and cell-contacts. mRNA expression of (a) sclerostin (SOST) and (b) connexin 43 (Cx43) in real-time RT-PCR of vertebral bodies Th10. Mice were immunized with testicular homogenate in adjuvant (EAO), adjuvant alone (adjuvant) or remained untreated (control). * p < 0.05; ** p < 0.01.
8. Biomechanical Properties
By a three-point bending test of the femorar, we objectified the consequences regarding bone metaboliomechanical bone properties due to the cellular and molecular alterations following EAO described before. The maximum load (Figure 12a) was significantly decreasmed in the EAO (8.234 ± 1.099 N) and adjuvant groups (10.392 ± 1.086 N) compared [13]to control (15.984 ± 0.665 N, p < 0.001 vs. EAO, p < 0.01 vs. Adj). The stiffness (Figure 12b) was significantly reduced in the EAO group (39.331 ± 7.355 N/mm, p < 0.05 vs. Cont 66.980 ± 4.208 N/mm) and almost significantly reduced in the adjuvant group (45.886 ± 8.284 N/mm, p = 0.055). Consequently, both immunized groups required less work to fracture compared to control (Figure 12c, EAO 4.277 ± 1.421 N/mm, Adj 5.566 ± 0.390 N/mm, Cont 12.520 ± 1.380 N/mm, p < 0.01 Cont vs. each).
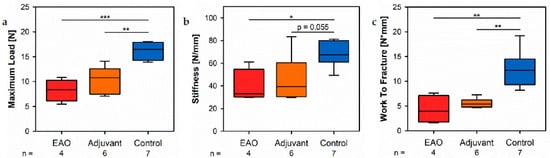

Figure 12. Biomechanical properties of the femora in the three-point bending test. Measurement of (a) maximum load, (b) stiffness and (c) work to fracture. Mice were immunized with testicular homogenate in adjuvant (EAO), adjuvant alone (adjuvant) or remained untreated (control). * p < 0.05; ** p < 0.01; *** p < 0.001.
9. Body Weight
Mice were weighed before the first immunization and 50 days after the first immunization in order to check their general health and well-being. Regarding the initial body weight (Table 1, Body weight before) as well as the body weight before euthanasia (Table 1, Body weight after) no differences between the investigated groups were noticed. Furthermore, all groups significantly gained body weight during the period of 50 days.
EAO is typically induced by active immunization with testicular homogenate in CFA [14]. The testicular homogenate is prepared from decapsulated testes collected from adult syngeneic mice and homogenized in sterile 0.9% NaCl at a ratio of 1:1. For immunization, testicular homogenate is mixed with CFA at a ratio of 1:1. In mice, four subcutaneous injections with a total volume of 200 μL (50 μL per injection site) per immunization are conducted dorsally under anaesthesia. Simultaneously, intraperitoneal injection of 100 ng Bordetella pertussis toxin in 100 μL Munõz Buffer (25 mM Tris, 0.5 M NaCl, 0.017% Triton X-100, pH 7.6) [15] is carried out in order to boost the immune reaction. In total, animals are immunized three times every 14 days. As an adjuvant control group, age-matched mice receive CFA mixed with 0.9% NaCl instead of testicular homogenate combined with Bordetella pertussis toxin following the same procedure. An additional control group of age-matched mice remains completely untreated. Depending on the purpose of the current study, euthanasia is conducted after individual time periods between 30 and 80 days after the first immunization [14].
Table 1. Body weights before the first immunization and 50 days after the first immunization.
| p-Values | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Statistic | Unit | EAO (n = 5) |
Adjuvant (n = 6) |
Control (n = 7) |
EAO vs. Adj. | EAO vs. Cont. | Adj. vs. Cont. |
| Body weight before | x¯ | |||||||
| ± SEM | g | 28.820 ± 1.233 | 27.283 ± 1.164 | 28.440 ± 0.680 | 0.978 | 1.000 | 1.000 |
| Body weight after | x¯ |
| ± SEM | g | 30.420 ± 1.283 | 29.783 ± 1.262 | 31.540 ± 0.963 | 1.000 | 1.000 | 0.938 | |
| p-values | before vs. after | 0.020 | 0.001 | 0.016 | ||||
References
- G. Haidl; J. P. Allam; H.-C. Schuppe; Chronic epididymitis: impact on semen parameters and therapeutic options. Andrologia 2008, 40, 92-96, 10.1111/j.1439-0272.2007.00819.x.Haidl, G.; Allam, J.P.; Schuppe, H.-C. Chronic epididymitis: Impact on semen parameters and therapeutic options. Andrologia 2008, 40, 92–96.
- H.-C. Schuppe; A. Meinhardt; J. P. Allam; M. Bergmann; W. Weidner; G. Haidl; Chronic orchitis: a neglected cause of male infertility?. Andrologia 2008, 40, 84-91, 10.1111/j.1439-0272.2008.00837.x.Schuppe, H.-C.; Meinhardt, A.; Allam, J.P.; Bergmann, M.; Weidner, W.; Haidl, G. Chronic orchitis: A neglected cause of male infertility? Andrologia 2008, 40, 84–91.
- Hans-Christian Schuppe; Adrian Pilatz; Hamid Hossain; Thorsten Diemer; Florian Wagenlehner; Wolfgang Weidner; Urogenital Infection as a Risk Factor for Male Infertility. Deutsches Aerzteblatt Online 2017, 114, 339-346, 10.3238/ARZTEBL.2017.0339.Schuppe, H.-C.; Pilatz, A.; Hossain, H.; Diemer, T.; Wagenlehner, F.; Weidner, W. Urogenital Infection as a Risk Factor for Male Infertility. Dtsch. Aerzteblatt Int. 2017, 114, 339–346.
- W. Weidner; A. Pilatz; Th. Diemer; H. C. Schuppe; A. Rusz; F. Wagenlehner; Male urogenital infections: impact of infection and inflammation on ejaculate parameters. World Journal of Urology 2013, 31, 717-723, 10.1007/s00345-013-1082-7.Weidner, W.; Pilatz, A.; Diemer, T.; Schuppe, H.C.; Rusz, A.; Wagenlehner, F. Male urogenital infections: Impact of infection and inflammation on ejaculate parameters. World J. Urol. 2013, 31, 717–723.
- G R Dohle; Giovanni M. Colpi; T B Hargreave; G K Papp; A Jungwirth; W Weidner; EAU Guidelines on Male Infertility. European Urology 2005, 48, 703-711, 10.1016/j.eururo.2005.06.002.Dohle, G.; Colpi, G.; Hargreave, T.; Papp, G.; Jungwirth, A.; Weidner, W. EAU Guidelines on Male Infertility. Eur. Urol. 2005, 48, 703–711.
- Monika Fijak; Eva Schneider; Jörg Klug; Sudhanshu Bhushan; Holger Hackstein; Gerhard Schuler; Malgorzata Wygrecka; Jörg Gromoll; Andreas Meinhardt; Testosterone Replacement Effectively Inhibits the Development of Experimental Autoimmune Orchitis in Rats: Evidence for a Direct Role of Testosterone on Regulatory T Cell Expansion. The Journal of Immunology 2011, 186, 5162-5172, 10.4049/jimmunol.1001958.Fijak, M.; Schneider, E.; Klug, J.; Bhushan, S.; Hackstein, H.; Schuler, G.; Wygrecka, M.; Gromoll, J.; Meinhardt, A. Testosterone Replacement Effectively Inhibits the Development of Experimental Autoimmune Orchitis in Rats: Evidence for a Direct Role of Testosterone on Regulatory T Cell Expansion. J. Immunol. 2011, 186, 5162–5172.
- Nour Nicolas; Julie A Muir; Susan Hayward; Justin L Chen; Peter Stanton; Paul Gregorevic; David M De Kretser; Kate Loveland; Sudhanshu Bhushan; Andreas Meinhardt; et al.Monika FijakMark P Hedger Induction of experimental autoimmune orchitis in mice: responses to elevated circulating levels of the activin-binding protein, follistatin. Reproduction 2017, 154, 293-305, 10.1530/rep-17-0010.Nicolas, N.; Muir, J.A.; Hayward, S.; Chen, J.L.; Stanton, P.G.; Gregorevic, P.; de Kretser, D.M.; Loveland, K.L.; Bhushan, S.; Meinhardt, A.; et al. Induction of experimental autoimmune orchitis in mice: Responses to elevated circulating levels of the activin-binding protein, follistatin. Reproduction 2017, 154, 293–305.
- Kenneth S.K. Tung; Mechanisms of autoimmune disease in the testis and ovary. Human Reproduction Update 1995, 1, 35-50, 10.1093/humupd/1.1.35.Tung, K.S.; Teuscher, C. Mechanisms of autoimmune disease in the testis and ovary. Hum. Reprod. Update 1995, 1, 35–50.
- Monika Fijak; Adrian Pilatz; Mark P Hedger; Nour Nicolas; Sudhanshu Bhushan; Vera Michel; Kenneth S K Tung; Hans-Christian Schuppe; Andreas Meinhardt; Infectious, inflammatory and ‘autoimmune’ male factor infertility: how do rodent models inform clinical practice?. Human Reproduction Update 2018, 24, 416-441, 10.1093/humupd/dmy009.Fijak, M.; Pilatz, A.; Hedger, M.P.; Nicolas, N.; Bhushan, S.; Michel, V.; Tung, K.S.K.; Schuppe, H.-C.; Meinhardt, A. Infectious, inflammatory and ‘autoimmune’ male factor infertility: How do rodent models inform clinical practice? Hum. Reprod. Update 2018, 24, 416–441.
- Diana Rucker; Shereen Ezzat; Anastasia Diamandi; Javad Khosravi; David A. Hanley; IGF-I and testosterone levels as predictors of bone mineral density in healthy, community-dwelling men. Clinical Endocrinology 2004, 60, 491-499, 10.1111/j.1365-2265.2004.02006.x.Rucker, D.; Ezzat, S.; Diamandi, A.; Khosravi, J.; Hanley, D.A. IGF-I and testosterone levels as predictors of bone mineral density in healthy, community-dwelling men. Clin. Endocrinol. 2004, 60, 491–499.
- A. D. Mooradian; J. E. Morley; S. G. Korenman; Biological Actions of Androgens. Endocrine Reviews 1987, 8, 1-28, 10.1210/edrv-8-1-1.Mooradian, A.D.; Morley, J.E.; Korenman, S.G. Biological actions of androgens. Endocr. Rev. 1987, 8, 1–28.
- Bart L. Clarke; Sundeep Khosla; Androgens and bone. Steroids 2009, 74, 296-305, 10.1016/j.steroids.2008.10.003.Clarke, B.L.; Khosla, S. Androgens and bone. Steroids 2009, 74, 296–305.
- Fabian Hemm; Monika Fijak; Jan Belikan; Marian Kampschulte; Thaqif El Khassawna; Adrian Pilatz; Christian Heiss; Katrin Lips; Bone Status in a Mouse Model of Experimental Autoimmune-Orchitis. International Journal of Molecular Sciences 2021, 22, 7858, 10.3390/ijms22157858.Francis, R.M.; Peacock, M.; Aaron, J.E.; Selby, P.L.; Taylor, G.A.; Thompson, J.; Marshall, D.H.; Horsman, A. Osteoporosis in hypogonadal men: Role of decreased plasma 1,25-dihydroxyvitamin D, calcium malabsorption, and low bone formation. Bone 1986, 7, 261–268.
- Nour Nicolas; Vera Michel; Sudhanshu Bhushan; Eva Wahle; Susan Hayward; Helen Ludlow; David M. De Kretser; Kate Loveland; Hans-Christian Schuppe; Andreas Meinhardt; et al.Mark P. HedgerMonika Fijak Testicular activin and follistatin levels are elevated during the course of experimental autoimmune epididymo–orchitis in mice. Scientific Reports 2017, 7, srep42391, 10.1038/srep42391.Rozenberg, S.; Bruyère, O.; Bergmann, P.; Cavalier, E.; Gielen, E.; Goemaere, S.; Kaufman, J.M.; Lapauw, B.; Laurent, M.R.; De Schepper, J.; et al. How to manage osteoporosis before the age of 50. Maturitas 2020, 138, 14–25.
- S Kohno; J A Munoz; T M Williams; C Teuscher; C C Bernard; K S Tung; Immunopathology of murine experimental allergic orchitis.. The Journal of Immunology 1983, 130, 2675-82.
More
