The term “inappropriate secretion of thyroid-stimulating hormone; IST” was proposed by Gershengorn and Weintraub in 1975. In a subsequent report, IST was described as a condition characterized by elevated serum levels of immunoreactive thyroid-stimulating hormone (TSH) in the presence of elevated free thyroid hormone concentrations. Similarly, the term "syndrome of IST (SITSH)" is widely used in Japan to refer to a closely related condition; however, unlike that for IST, an elevated serum free triiodothyronine concentration is not a requisite criterion for SITSH diagnosis. IST or SITSH is an important indicator of resistance to thyroid hormone β (RTHβ) caused by germline mutations in genes encoding thyroid hormone receptor β (TRβ) and TSH-secreting pituitary adenoma.
- thyroid function
- inappropriate secretion of thyroid-stimulating hormone (IST)
- syndrome of inappropriate secretion of thyroid-stimulating hormone (SITSH)
- genuine IST
1. Introduction
Circulating concentrations of thyroid-stimulating hormone (TSH) and other thyroid hormones are tightly regulated in healthy individuals [1]. The liganded thyroid hormone receptor (TR) negatively regulates the synthesis and secretion of TSH in pituitary thyrotrophs, resulting in drastic decreases in serum TSH concentrations [2]. For example, the most common forms of hyperthyroidism, including Graves’ disease and toxic multinodular goiter, demonstrate elevated thyroid hormone levels with suppressed TSH concentrations.
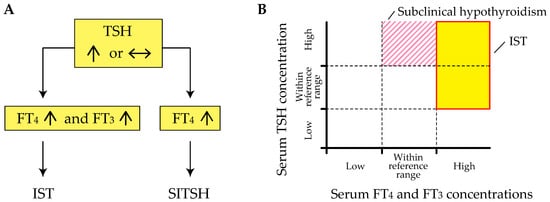
Inappropriate secretion of TSH (IST) is characterized by the laboratory findings of elevated free thyroxine (T4) and triiodothyronine (T3) concentrations in the presence of detectable TSH concentrations [3][4], contrary to what happens in the most common forms of hyperthyroidism (Figure 1 A). This abnormal pattern in the thyroid function test is an important hallmark of genetic and acquired disorders of the hypothalamus-pituitary-thyroid axis (HPT-axis), including resistance to thyroid hormone β (RTHβ) caused by germline mutations in genes encoding the β isoform of the TR (TRβ) and TSH-secreting pituitary adenoma (TSHoma). Although previous review articles have sufficiently investigated several conditions associated with IST, little information is available on systematic pathophysiological aspects of IST in previous review articles. In addition, a recent study has revealed that diagnostic delay and inappropriate treatments (e.g., pituitary surgeries in patients with RTHβ or thyroid ablation in patients with TSHoma) are still provided in several cases with thyroid hormone profiles suggestive of IST [5].
2. Definition of IST
The term “IST” was proposed by Gershengorn and Weintraub in 1975 [6]. In a subsequent report, IST was described as a condition characterized by elevated serum levels of immunoreactive TSH in the presence of elevated free thyroid hormone concentrations [3]. However, it has been suggested that TSH secretion is compensatory and appropriate in RTHβ, because the response to thyroid hormones in peripheral tissues is reduced due to impairment of the TR [7]. Beck-Peccoz et al. also suggested that the definition of “IST” appears inadequate, as it does not reflect the pathophysiological events underlying TSHoma and RTHβ [4]. Therefore, the authors proposed the term “central hyperthyroidism” instead of IST, because TSH itself is responsible for the hyperstimulation of the thyroid gland and the consequent thyrotoxicosis.
The term “syndrome of inappropriate secretion of thyroid-stimulating hormone (SITSH)” has been widely used in journal articles and textbooks written by Japanese researchers since the late 1980s. Based on the 2016 Japan Thyroid Association (JTA) guidelines for the diagnosis of RTHβ, SITSH is defined as being characterized by elevated free T4 and normal-to-elevated TSH concentrations [8] (Figure 1 A). An elevated serum free T3 concentration is not a requisite criterion for SITSH diagnosis. TIn this study, the author conducted a MEDLINE search using the keyword “SITSH” to retrieve relevant articles published between 1975 and 2020. The literature search yielded 30 articles, of which 28 were published in Japanese institutions. The similarity in the spelling of SITSH to that of SIADH (syndrome characterized by the inappropriate secretion of antidiuretic hormone) might be responsible for the wide use of SITSH in Japan.
Physicians must keep in mind that elevated thyroid hormone concentrations are requisite for the diagnosis of IST, because at least two previous studies defined the laboratory findings of inappropriately elevated TSH concentration concurrent with non-elevated thyroid hormone concentrations as the criterion for IST [9][10]. Tyrosine kinase inhibitor therapy, specifically therapy using axitinib, can reportedly cause inappropriately elevated TSH concentrations despite the thyroid hormone concentrations being within the reference range [11]. Similar patterns of thyroid function profiles have been reported in patients with macro-thyrotropin (macro-TSH) [12][13][14], or in those with RTHβ and Hashimoto’s thyroiditis [15]. These findings of thyroid function tests meet the definition of subclinical hypothyroidism but not IST (Figure 1 B).
3. Pathophysiology of IST
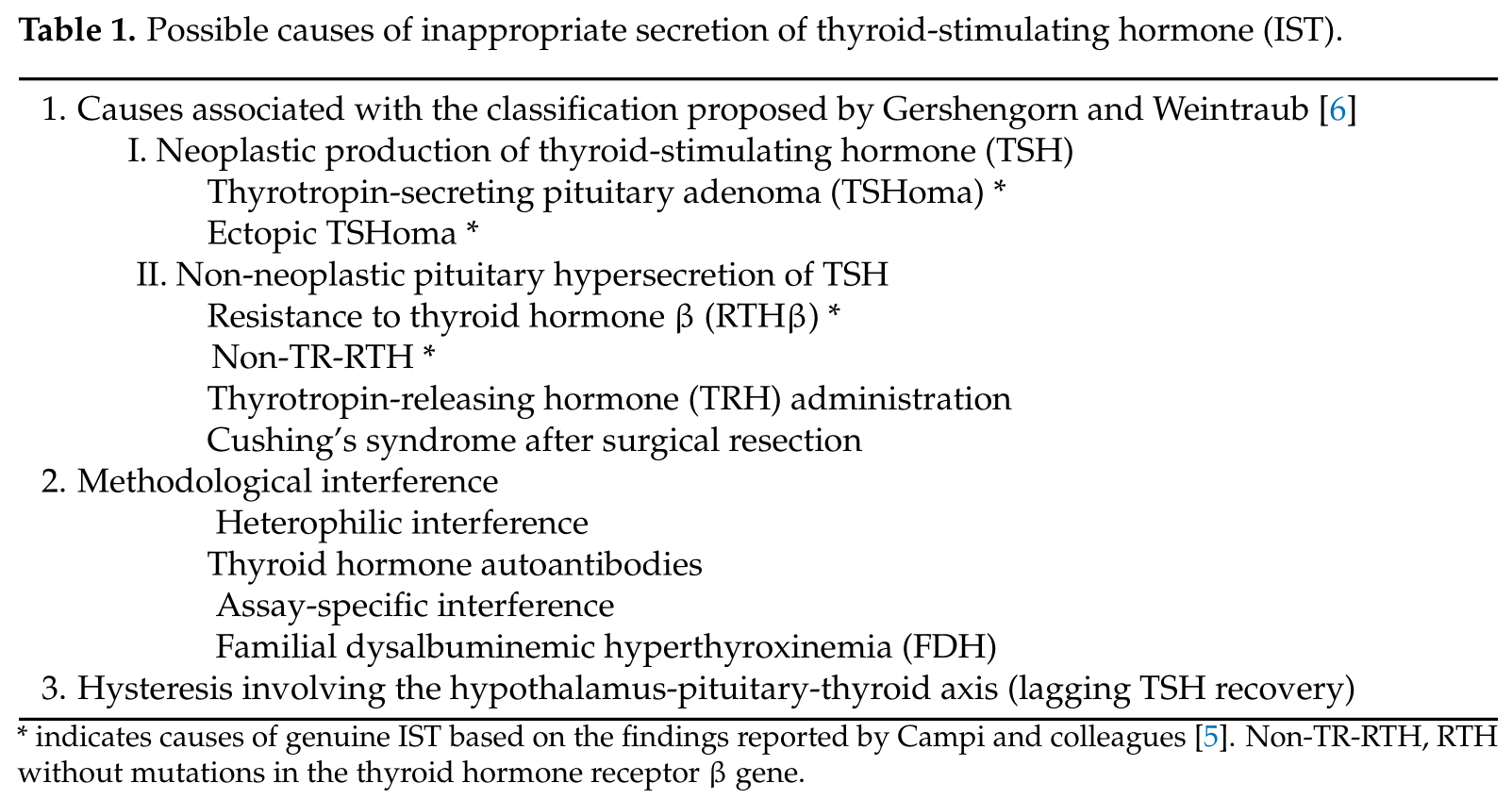
Gershengorn and Weintraub proposed a classification of ISufT in terms of its pathogenesis [6]. The major classificatient evidence has been accumulated for several conditions associated with Ion was dependent on whether IST was either associated with a neoplasm (class I) or was not (class II). Neoplastic IST (class I) was further classified based on tumor location (i.e., class I-A; pituitary tumors and class I-B; non-pituitary tumors (ectopic production)). Non-neoplastic ISTs (class II) were classified into three groups: RTH (class II-A), abnormal stimulation of TSH secretion by thyrotropin-releasing hormone (TRH) or other stimulators (class II-B), and defective suppression of TSH secretion by somatostatin, dopamine, or other suppressors (class II-C).
STecond, as summarized in Table 1. In a recent study reported by Campi and colleaguesRefetoff et al. proposed the term impaired sensitivity to thyroid hormone (ISTH), which includes RTHβ, non-TR-RTH, selenocysteine insertion sequence-binding protein 2 (SBP2) defect, monocarboxylate transporter 8 (MCT8) defect, and RTHα caused by germline mutations in the genes encoding the α isoform of the TR (TRα) [516],. Among the term “genuine IST” was used to indicate the etiologym, the hormonal profile of non-TR-RTH fulfills the definition of IST. In contrast, thyroid function profiles of SBP2 defects, MCT8 defects, and RTHα do not meet the definition of IST, includinbecause they are associated with high serum T4 or T3 only concurrent with non-suppressed TSH concentrations [7][17].
Second, in the phenomenon of hysteresis in the HPT-axis (lagging TSHoma, RTHβ, and a syndrome clinica recovery), persistent TSH elevation (or suppression) with consequent lagging of thyrotroph recovery following hypothyroidism (or thyrotoxicosis) has been documented for many years [18]. Dellay and biochemically inded response of thyrotrophs could result in non-suppression of TSH despite elevated thyroid hormones in several conditions (e.g., pre-existinguishable from RTHβ but without mutations in the TRβ gene (non-TR-RTH) hypothyroidism secondary to chronic thyroiditis and subsequent onset of destructive thyroiditis, or levothyroxine replacement therapy associated with poor compliance). Here, we focus on the potential mechanism of this condition in terms of epigenetic regulation.
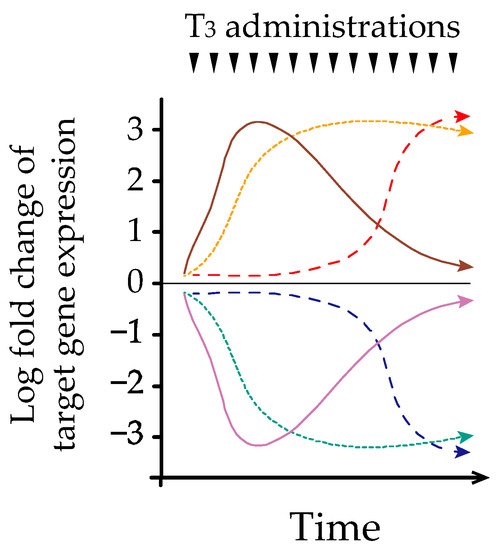
GHysteresis refers to the phengorn andomenon observed in systems exhibiting a memory effect, such that the response to an input is delayed by a lag time W[19]. Melvintra et al. first described hysteresis involving the HPT-axis in 2007 [20]. However, the precise mechanism ubnderlying this phenomenon remains proposed a classification of IST in termoorly understood. We and others have identified early, late, and sustained patterns of hepatic transcriptional responses after acute and/or chronic thyroid hormone treatment in adult murine models [21][22] (Figure 2 ). Further analysis of epits pathogenesisgenetic regulation demonstrated that histone H3 acetylation at lysines 9 and 14 (H3K9/K14ac) was associated with acute thyroid hormone stimulation, [6].whereas The major classificationistone H3 trimethylation at lysine 4 (H3K4me3) is associated with chronic stimulation [22]. Similarly, Umezawas dependent on whether IST was either associ et al. reported that chronic thyroid hormone treatment in a rat pituitary cell line caused sustained suppression of TRH, which was associated with a prolonged decrease in H3K4me3 and only a transient reduction in H3K9/K14ac [23]. Based on ted with a neoplasm (class I) or was not (class II)hese findings, different chromatin modifications of target genes could play an important role in regulating transcription during acute and chronic thyroid hormone treatments. We have also reported that thyroid hormones reduce hepatic TRβ protein expression in a time-dependent manner [24]. NTakeoplastic IST (class I) was fn together, time-dependent changes in transcriptional mechanisms may partly explain the hysteresis of thyroid hormone-responsive genes. Further classified based ostudies are needed to clarify the mechanism of hysteresis associated with the HPT-axis, especially using the pituitary and hypothalamic models.
4. Differential Diagnosis of IST
Once tumor location (he existence of genuine IST is confirmed, it is important to differentiate between RTHβ, non-TR-RTH, and TSHoma [5]. The possi.ble., class I-A; presence of neurological signs and symptoms associated with the pressure effects of the pituitary tumors and class I-B; non-adenoma or clinical features of concomitant hypersecretion of other pituitary tumors (ectopic production)). Non-neophormones supports the probability of pituitary TSHoma occurrence. Because no differences in the basal concentrations of TSH and free thyroid hormones have been reported [25][26], several diastic ISTs (class II) were classified intgnostic steps are required. First, investigating the presence of IST in the family members of a patient can provide important clues for the diagnosis of RTHβ, because no familial cases of TSHoma have been documented [27]. Notably, three groups: RTH (class II-A), abnormal e frequency of de novo RTHβ cases is approximately 20% [7]. Second, genetic testing and imulation of TSH secretion by thyrotropin-releasing hoaging studies are useful to confirm the diagnosis of RTHβ and TSHoma, respectively. However, differential diagnosis may be challenging if the pituitary adenoma is very small or missing in patients with the absence of mutations in the TRβ gene. Patients with IST and negative pituitary findings may develop TSHoma during long-term follow-up, as Campi et al. recently reported that pituitary MRI returned negative results in 6 of 26 cases with TSHoma [5]. The differmential diagnone (TRH) or other stimulators (class II-B), ansis between non-TR-RTH and TSHoma is important in some patients with pituitary adenoma, because approximately 10% of the healthy adult population has pituitary abnormalities on MRI scans that are compatible with the diagnosis of asymptomatic pituitary adenomas [28]. A recent study defective surevealed that 11 out of 45 patients with RTHβ have small pituitary lesions [5].
Several pproceduression of TSH secretion by somatostatin for the differential diagnosis of problematic cases of IST have been proposed, including dynamic testing and determination of biochemical parameters. In line with dynamic testing, it has been suggested that the combination of dynamic testing could increase the accuracy of the diagnostic workup [26][29]. First, the TRH stimulation test dopamine, or oemonstrated normal or increased TSH responses (similar to hypothyroid) in most patients with RTHβ or non-TR-RTH, whereas the vast majority of patients with TSHoma do not respond to TRH [25][27][30]. Second, ther T3-suppressors (clasion test (fixed dose of 75–100 μg of liothyronine (L-T3) administered for 7–10 days II-C[31][27]).
W is useful to evaluate the presen Gershence of TSHoma, because TSH concentrations after T3-suppression are reportedly higher in cases with TSHoma than in cases with RTHβ [5][32]. Third, a standardized diagnorn astic protocol using incremental doses of L-T3 [33], instead Weintraub proposed their clof a fixed dose is important for the diagnosis of RTHβ and non-TR-RTH, wherein blunted TSH suppression after TRH administration and diminished metabolic response are typically observed [7]. However, dynamic tessifications, clinical entitiests using fixed or incremental doses of L-T3 are time-consuming and contraindicated in elderly patients or in those with coronary heart disease [32]. Fofurth, I-A and II-A had been documented, but otheattention has been paid to dynamic tests using somatostatin analogs (SSAs). Mannavola identified a significant reduction in serum thyroid hormone concentrations after the long-term administration of SSA in patients with TSHoma but not in those with RTHβ [34]. More classes were only postulatedrecently, Han et al. demonstrated that even a short-term dynamic test using short-acting SSAs may provide an alternative diagnostic approach for TSHoma [635].
The Smeasubsequent sturement of biochemical parameters is also important to reach a differential diagnosis in problematic cases of IST. Previous studies have revealed that several condrum glycoprotein hormone α-subunit (α-GSU) concentrations are elevated in more than 70% of patients with TSHoma, particularly in those with a macroadenoma [36], whereas this parameter is withions or n the reference range in most patients with RTHβ or non-TR-RTH [25][27]. As wituations are associah other biochemical parameters, measurement of metabolic markers of thyroid hormone action can be helpful in determining peripheral resistance to thyroid hormones. Among these markers, serum sex hormone-binding globulin (SHBG) and carboxy-terminal cross-linked telopeptide of type-I collagen (ITCP) appear to be useful for differential diagnoses [26][29]. Othed with IST, as propr circulating markers, including alkaline phosphatase, brain natriuretic peptide, cholesterol, creatine kinase, and ferritin are of limited help in assessing the impact of thyroid hormones on peripheral tissues [25][26][37].
For a better undersed by the authorstanding of the differential diagnosis of problematic cases of IST (or SITSH), several flowcharts have been proposed [5][8][26][29].
References
- Hollenberg, A.N. Regulation of Thyrotropin Secretion. In The Thyroid A Fundamental and Clinical Text, 10th ed.; Braverman, L.E., Cooper, D.S., Eds.; Lippincott Williams & Wilkins, Philadelphia, PA , USA, 2013; pp. 169–182.Hollenberg, A.N. Regulation of Thyrotropin Secretion. In The Thyroid A Fundamental and Clinical Text, 10th ed.; Braverman, L.E., Cooper, D.S., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 169–182.
- Sasaki, S.; Matsushita, A.; Kuroda, G.; Nakamura, H.M.; Oki, Y.; Suda, T. The Mechanism of Negative Transcriptional Regulation by Thyroid Hormone: Lessons from the Thyrotropin β Subunit Gene. Vitam. Horm. 2018, 106, 97–127.
- Weintraub, B.; Gershengorn, M.; Kourides, I.; Fein, H. Inappropriate Secretion of Thyroid-Stimulating Hormone. Ann. Intern. Med. 1981, 95, 339–351.
- Beck-Peccoz, P.; Brucker-Davis, F.; Persani, L.; Smallridge, R.C.; Weintraub, B.D. Thyrotropin-Secreting Pituitary Tumors. Endocr. Rev. 1996, 17, 610–638.
- Campi, I.; Covelli, D.; Moran, C.; Fugazzola, L.; Cacciatore, C.; Orlandi, F.; Gallone, G.; Chatterjee, K.; Beck-Peccoz, P.; Persani, L. The Differential Diagnosis of Discrepant Thyroid Function Tests: Insistent Pitfalls and Updated Flow-Chart Based on a Long-Standing Experience. Front. Endocrinol. Lausanne 2020, 11, 432.
- Gershengorn, M.C.; Weintraub, B.D. Thyrotropin-Induced Hyperthyroidism Caused by Selective Pituitary Resistance to Thy-roid Hormone. A New Syndrome of "Inappropriate Secretion of TSH". J. Clin. Investig. 1975, 56, 633–642.Gershengorn, M.C.; Weintraub, B.D. Thyrotropin-Induced Hyperthyroidism Caused by Selective Pituitary Resistance to Thyroid Hormone. A New Syndrome of “Inappropriate Secretion of TSH”. J. Clin. Investig. 1975, 56, 633–642.
- Dumitrescu, A.M.; Refetoff, S. Impaired Sensitivity to Thyroid Hormone: Defects of Transport, Metabolism and Action. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., Kaltsas, G.; et al. Eds.; MDText.com, Inc.: South Dartmouth, MA , USA, 2015.Dumitrescu, A.M.; Refetoff, S. Impaired Sensitivity to Thyroid Hormone: Defects of Transport, Metabolism and Action. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., Kaltsas, G., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2015.
- Japan Thyroid Association. Diagnosis of the Resistance to Thyroid Hormone Beta (RTHβ). 2016. Available online: http://www.japanthyroid.jp/doctor/img/hormone03.pdf (accessed on 28 April 2021).
- Gessl, A.; Blueml, S.; Bieglmayer, C.; Marculescu, R. Anti-Ruthenium Antibodies Mimic Macro-TSH in Electrochemilumines-cent Immunoassay. Clin. Chem. Lab. Med. 2014, 52, 1589–1594.Gessl, A.; Blueml, S.; Bieglmayer, C.; Marculescu, R. Anti-Ruthenium Antibodies Mimic Macro-TSH in Electrochemiluminescent Immunoassay. Clin. Chem. Lab. Med. 2014, 52, 1589–1594.
- Kara, C.; Ocal, G.; Berberoğlu, M.; Siklar, Z.; Adiyaman, P. Persistently Raised Thyroid Stimulating Hormone in Adequately Treated Congenital Hypothyroidism on Long-Term Follow-Up. J. Pediatr. Endocrinol. Metab. 2008, 21, 251–256.
- Ohba, K.; Takayama, T.; Matsunaga, H.; Matsushita, A.; Sasaki, S.; Oki, Y.; Ozono, S.; Nakamura, H. Inappropriate Elevation of Serum Thyrotropin Levels in Patients Treated with Axitinib. Thyroid 2013, 23, 443–448.
- Sakai, H.; Fukuda, G.; Suzuki, N.; Watanabe, C.; Odawara, M. Falsely Elevated Thyroid-Stimulating Hormone (TSH) Level due to Macro-TSH. Endocr. J. 2009, 56, 435–440.
- Hattori, N.; Ishihara, T.; Matsuoka, N.; Saito, T.; Shimatsu, A. Anti-Thyrotropin Autoantibodies in Patients with Mac-ro-Thyrotropin and Long-Term Changes in Macro-Thyrotropin and Serum Thyrotropin Levels. Thyroid 2017, 27, 138–146.Hattori, N.; Ishihara, T.; Matsuoka, N.; Saito, T.; Shimatsu, A. Anti-Thyrotropin Autoantibodies in Patients with Macro-Thyrotropin and Long-Term Changes in Macro-Thyrotropin and Serum Thyrotropin Levels. Thyroid 2017, 27, 138–146.
- Ohba, K.; Maekawa, M.; Iwahara, K.; Suzuki, Y.; Matsushita, A.; Sasaki, S.; Oki, Y.; Nakamura, H. Abnormal Thyroid Hor-mone Response to TRH in a Case of Macro-TSH and the Cut-Off Value for Screening Cases of Inappropriate TSH Elevation. Endocr. J. 2020, 67, 125–130.Ohba, K.; Maekawa, M.; Iwahara, K.; Suzuki, Y.; Matsushita, A.; Sasaki, S.; Oki, Y.; Nakamura, H. Abnormal Thyroid Hormone Response to TRH in a Case of Macro-TSH and the Cut-Off Value for Screening Cases of Inappropriate TSH Elevation. Endocr. J. 2020, 67, 125–130.
- Fukata, S.; Brent, G.A.; Sugawara, M. Resistance to Thyroid Hormone in Hashimoto's Thyroiditis. N. Engl. J. Med. 2005, 352, 517–518.Fukata, S.; Brent, G.A.; Sugawara, M. Resistance to Thyroid Hormone in Hashimoto’s Thyroiditis. N. Engl. J. Med. 2005, 352, 517–518.
- Refetoff, S.; Bassett, J.H.; Beck-Peccoz, P.; Bernal, J.; Brent, G.; Chatterjee, K.; De Groot, L.J.; Dumitrescu, A.M.; Jameson, J.L.; Kopp, P.A.; et al. Classification and Proposed Nomenclature for Inherited Defects of Thyroid Hormone Action, Cell Transport, and Metabolism. Eur. Thyroid J. 2014, 3, 7–9.
- Onigata, K.; Szinnai, G. Resistance to Thyroid Hormone. Endocr. Dev. 2014, 26, 118–129.
- Leow, M.K. A Review of the Phenomenon of Hysteresis in the Hypothalamus-Pituitary-Thyroid Axis. Front. Endocrinol. Lausanne 2016, 7, 64.
- Ewing, J.A. On Hysteresis in the Relation of Strain to Stress; British Association Reports; British Association: London, UK, 1889; pp. 502–504. Available online: https://archive.org/details/reportofbritisha90brit/page/502/mode/2up (accessed on 28 April 2021).
- Leow, M.K. A Mathematical Model of Pituitary-Thyroid Interaction to Provide an Insight into the Nature of the Thyrotropin--Thyroid Hormone Relationship. J. Theor. Biol. 2007, 248, 275–287.
- Flores-Morales, A.; Gullberg, H.; Fernandez, L.; Ståhlberg, N.; Lee, N.H.; Vennström, B.; Norstedt, G. Patterns of Liver Gene Expression Governed by TRbeta. Mol. Endocrinol. 2002, 16, 1257–1268.
- Ohba, K.; Leow, M.K.; Singh, B.K.; Sinha, R.A.; Lesmana, R.; Liao, X.H.; Ghosh, S.; Refetoff, S.; Sng, J.C.; Yen, P.M. Desensitization and Incomplete Recovery of Hepatic Target Genes After Chronic Thyroid Hormone Treatment and Withdrawal in Male Adult Mice. Endocrinology 2016, 157, 1660–1672.
- Umezawa, R.; Yamada, M.; Horiguchi, K.; Ishii, S.; Hashimoto, K.; Okada, S.; Satoh, T.; Mori, M. Aberrant Histone Modifications at the Thyrotropin-Releasing Hormone Gene in Resistance to Thyroid Hormone: Analysis of F455S Mutant Thyroid Hormone Receptor. Endocrinology 2009, 150, 3425–3432.
- Ohba, K.; Sinha, R.A.; Singh, B.K.; Iannucci, L.F.; Zhou, J.; Kovalik, J.P.; Liao, X.H.; Refetoff, S.; Sng, J.C.G.; Leow, M.K.; et al. Changes in Hepatic TRβ Protein Expression, Lipogenic Gene Expression, and Long-Chain Acylcarnitine Levels During Chronic Hyperthyroidism and Triiodothyronine Withdrawal in a Mouse Model. Thyroid 2017, 27, 852–860.
- Macchia, E.; Lombardi, M.; Raffaelli, V.; Piaggi, P.; Macchia, L.; Scattina, I.; Martino, E. Clinical and Genetic Characteristics of a Large Monocentric Series of Patients Affected by Thyroid Hormone (Th) Resistance and Suggestions for Differential Diagnosis in Patients without Mutation of Th Receptor β. Clin. Endocrinol. 2014, 81, 921–928.
- Beck-Peccoz, P.; Lania, A.; Beckers, A.; Chatterjee, K.; Wemeau, J.L. 2013 European Thyroid Association Guidelines for the Diagnosis and Treatment of Thyrotropin-Secreting Pituitary Tumors. Eur. Thyroid J. 2013, 2, 76–82.
- Beck-Peccoz, P.; Persani, L.; Lania, A. Thyrotropin-Secreting Pituitary Adenomas. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2019.
- Hall, W.A.; Luciano, M.G.; Doppman, J.L.; Patronas, N.J.; Oldfield, E.H. Pituitary Magnetic Resonance Imaging in Normal Human Volunteers: Occult Adenomas in the General Population. Ann. Intern. Med. 1994, 120, 817–820.
- Tjörnstrand, A.; Nyström, H.F. Diagnosis of Endocrine Disease: Diagnostic Approach to TSH-Producing Pituitary Adenoma. Eur. J. Endocrinol. 2017, 177, R183–R197.
- Yamada, S.; Fukuhara, N.; Horiguchi, K.; Yamaguchi-Okada, M.; Nishioka, H.; Takeshita, A.; Takeuchi, Y.; Ito, J.; Inoshita, N. Clinicopathological Characteristics and Therapeutic Outcomes in Thyrotropin-Secreting Pituitary Adenomas: A Single-Center Study of 90 Cases. J. Neurosurg. 2014, 121, 1462–1473.
- Murata, Y. Diagnostic Criteria Making of the Resistance to Thyroid Hormone. J. Jpn. Thyroid Assoc. 2012, 3, 10–14. (In Japanese)
- Beck-Peccoz, P.; Persani, L.; Mannavola, D.; Campi, I. Pituitary tumours: TSH-Secreting Adenomas. Best. Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 597–606.
- Refetoff, S.; Weiss, R.E.; Usala, S.J. The Syndromes of Resistance to Thyroid Hormone. Endocr. Rev. 1993, 14, 348–399.
- Mannavola, D.; Persani, L.; Vannucchi, G.; Zanardelli, M.; Fugazzola, L.; Verga, U.; Facchetti, M.; Beck-Peccoz, P. Different Responses to Chronic Somatostatin Analogues in Patients with Central Hyperthyroidism. Clin. Endocrinol. 2005, 62, 176–181.
- Han, R.; Shen, L.; Zhang, J.; Xie, J.; Fang, W.; Sun, Q.; Bian, L.; Zhou, Y.; Wang, S.; Ning, G.; et al. Diagnosing Thyrotropin-Secreting Pituitary Adenomas by Short-Term Somatostatin Analogue Test. Thyroid 2020, 30, 1236–1244.
- Socin, H.V.; Chanson, P.; Delemer, B.; Tabarin, A.; Rohmer, V.; Mockel, J.; Stevenaert, A.; Beckers, A. The Changing Spectrum of TSH-Secreting Pituitary Adenomas: Diagnosis and Management in 43 Patients. Eur. J. Endocrinol. 2003, 148, 433–442.
- Ohba, K.; Okada, E.; Goto, Y.; Suzuki, S.; Machii, M.; Nonaka, D.; Matsushita, A.; Sasaki, S.; Suda, T.; Oki, Y.; et al. Influence of Thyroid Dysfunction on Brain Natriuretic Peptide Level in Health Examination Participants. Endocr. J. 2020, 67, 449–454.
